What exactly are the physical laws that transmit the DNA codes through molecular messengers with remarkable accuracy and lead to the self-assembly of our bodies?
As Raghuveer Parthasarathy writes in So Simple a Beginning: How Four Physical Principles Shape Our Living World:
In living cells as well as in sterile test tube solutions, single strands of DNA will spontaneously wrap themselves into a double helix if their nucleotides complement each other [that is, if their charge structures allow them to share a proton]. No external scaffolding or microscopic ropes and pulleys are required: the DNA contains within itself the mechanism of its organization, highlighting a theme of self-assembly that surfaces repeatedly in our explorations of life … The double-stranded helix describes how DNA is situated in space; the size, shape, stiffness, and electrical charge of double-stranded DNA govern its packaging in cells and the readout of information it contains.
Some of the keys to self-assembly are heat, which makes small particles vibrate, and also the different types of electrical charges, which make different particles bind together when they meet each other. The great physicist Richard Feynman wonderfully describes how charged atoms can be bumped around when heat gives them the energy to move around more quickly, which then allows them to fuse together in different ways when the forces of attraction overcome the forces of repulsion, in this video here, which you should watch before reading on.
Proteins are the master messengers of our bodies, performing a large array of tasks, like catalyzing metabolic reactions, replicating DNA, and responding to stimuli. So eat your protein! As Parthasarathy writes:
At the core of nearly every action, every task, and every event in your body is a protein. Proteins in red blood cells soak up oxygen from the air you breathe. Proteins tug on other proteins to contract your muscles … Proteins in your eyes capture light and trigger electrical impulses, while other proteins open and close gates that send these impulses to your brain. Many kinds of proteins are inside every cell, and many are outside as well, making up, for example, the elastic matrix of your flesh. So: What are proteins? Like DNA, a protein is a molecule composed of a chain of simple units. In DNA, these units are any of four nucleotides. In proteins, these units are any of 20 amino acids. Double-stranded DNA, regardless of its nucleotide sequence, adopts a double-helical structure. In contrast, proteins have structures that are determined by their particular amino acid sequences. Every distinct protein has a different pattern of amino acids and therefore a different three-dimensional shape.
So how do molecules form into these protein messengers all by themselves? Through the amazing natural forces of attraction:
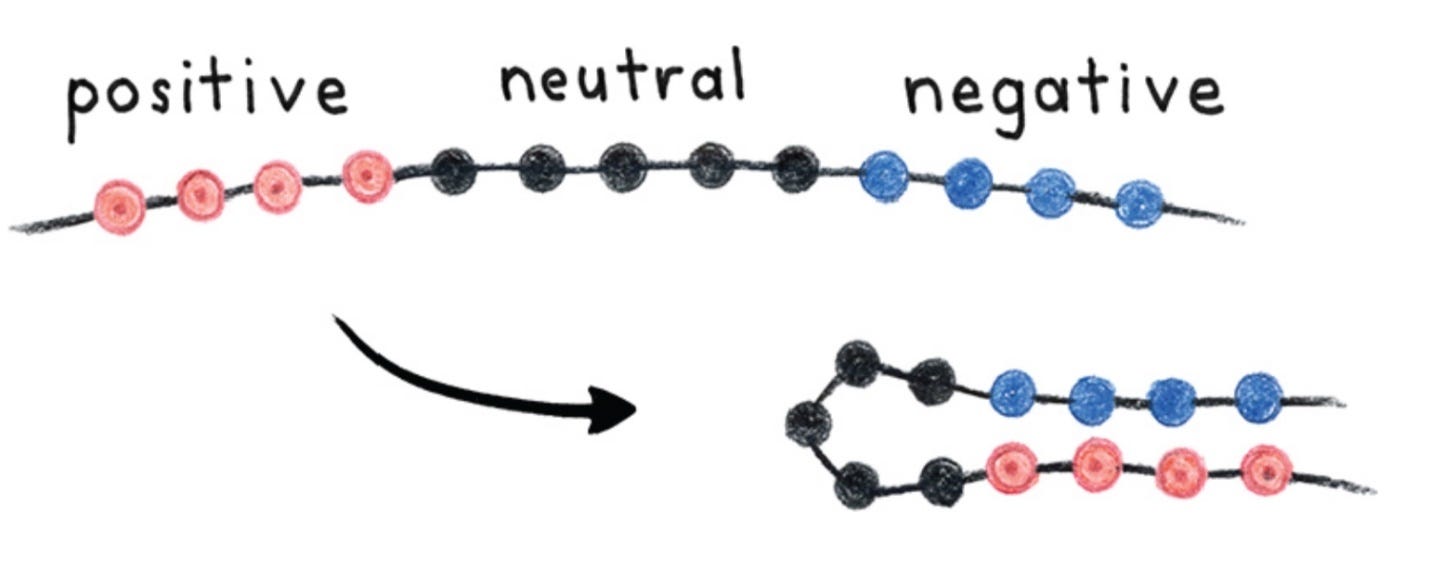
Each of the 20 amino acids has particular physical properties. Some have positive electrical charge; some are negative; some are neutral. Some are large; some are small. Some are greasy (“hydrophobic”) and prefer to separate from water; some are “hydrophilic” and mix well with water. Imagine a protein with several positive amino acids in a row (red circles in the illustration), followed by a string of neutral hydrophilic amino acids (black circles), and then several negative amino acids (blue circles). Opposite charges attract; so left to its own devices, the protein folds to bring together the contrasting ends.
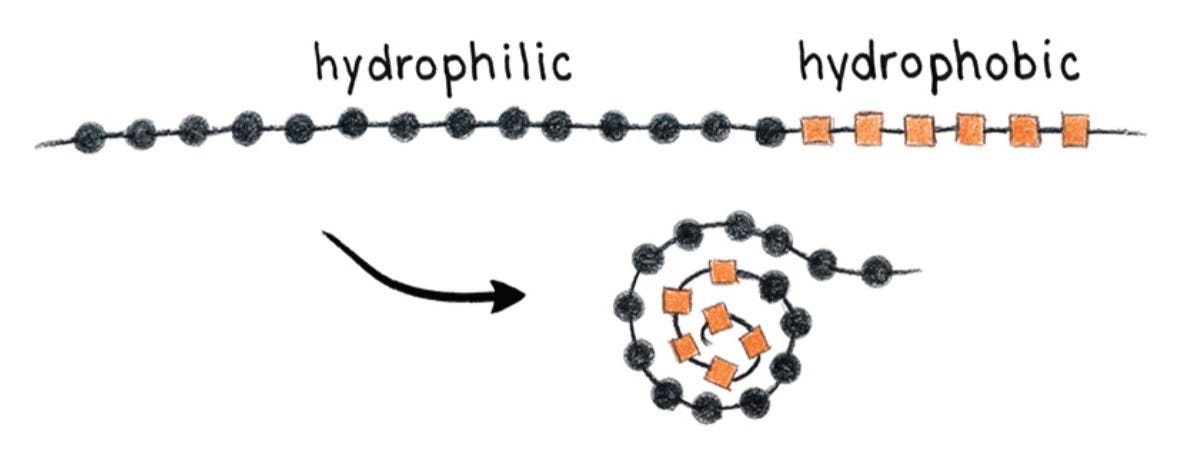
Or imagine a protein with amino acids that are hydrophobic (orange squares) and hydrophilic (black circles). The protein is surrounded by water—water makes up the majority of the cellular interior—and will fold to bury the hydrophobic bits in the center to be surrounded by their water-loving colleagues.
Every protein we’ve described above, and tens of thousands of others, folds within a fraction of a second into a three-dimensional form, bypassing the innumerable pitfalls and dead ends of shapes that don’t quite satisfy the interactions their component parts prefer. This is an amazing feat—like a piece of paper spontaneously folding itself into a perfect origami sculpture. What’s more, for the vast majority of proteins the sculpture is uniquely determined by the amino acid sequence. In other words, a given sequence always folds into the same shape.
Relatively simple physical principles also self-assemble the membranes of all our cells:
You began as a single cell, a fertilized egg. This cell divided in two, these two into four, and after many more divisions, rearrangements, and changes of shape, you developed a body composed of tens of trillions of cells. Every one of these cells has a membrane at its edge. This membrane doesn’t just serve as a boundary between inside and outside, but forms an arena where the cell grabs onto its surroundings, traffics chemicals, and exchanges signals with its neighbors … At the core of every cellular membrane is a sheet just a few billionths of a meter thick made of molecules called lipids … To understand the properties of membranes and how they emerge, let’s first look at something more familiar. Oil and water don’t mix. The oil in a shaken bottle of vinaigrette salad dressing quickly coalesces into droplets. Left alone, oil molecules associate with other oil molecules and water molecules with water molecules … [S]ubstances such as oils and fats that separate from water are called hydrophobic (“water-fearing”); substances such as sugar and vinegar that mix with water are hydrophilic (“water-loving”). Lipids are both hydrophobic and hydrophilic. Each lipid molecule has a “head” that likes water and a “tail” that doesn’t. The tail typically consists of two chains, each of which chemically resembles an oil molecule. There are other familiar substances that also have these amphiphilic (“loving both”) tendencies: every soap molecule also has a hydrophilic head and a hydrophobic tail, typically just one chain, which together let the soap cling to greasy dirt as well as to the water that washes it away. Lipids in water struggle with the contradiction imposed by their structure: their heads are happy but their tails are not. They therefore spontaneously associate with one another to shield their tails from the water, forming a two-molecule-thick sheet. This “lipid bilayer” forms the basis of all cellular membranes … [T]he lipids arrange themselves into a bilayer simply because that shape minimizes contact between the hydrophobic chains and water. The cell doesn’t need genes to tell lipids to form bilayers; this is simply what lipids do … As with the folding of proteins, we see the powerful general principle of self-assembly at work: simple physical concerns can orchestrate the formation of structure, allowing molecules to organize themselves. Harnessing self-assembly is not only useful for nature but also inspirational for those of us who study nature, showing that life need not be as complicated as it may initially seem—that physical simplicity may underlie biological complexity.
So really, we’re all like self-assembling origami, with different forces of electrical attraction or repulsion at the corners of each fold, making the biological paper that makes us up fold where it does.
Where did our organic origami blueprints come from? As Parthasarathy explains:
[T]he proteins that actually exist in the real world aren’t random, but rather are those that have been selected by four billion years of evolution. Organisms that encode amino acid sequences that don’t fold into unique shapes are plagued by dysfunctional and perhaps even harmful proteins, and hence are less likely to live and reproduce. Those that persist are those that encode amino acid sequences with a clear, unique path to three-dimensional structure.
And as Johnjoe McFadden and Jim Al-Khalili write in their book Life on the Edge: The Coming Age of Quantum Biology, “These nanomachines of nature are performing, at a molecular level, a carefully choreographed dance whose actions have been precision engineered by millions of years of natural selection to manipulate the motion of the fundamental particles of matter.”
Billions of years of evolution has resulted in organic molecules so complex in their interaction, that even the most powerful computers today can’t figure all of them out. As Parthasarathy writes:
In principle, since we understand the physics of electrical forces and hydrophobic and hydrophilic interactions, we should be able to simply plug the amino acid sequence into a computer program, easy enough to write, that grinds through the requisite calculations, stopping when it has found the optimal folding of the molecular chain. In practice, however, the number of possible configurations is so enormous that even the fastest computers struggle to explore them all … It is humbling to consider that the proteins themselves have solved the protein folding problem, shaping their structures within fractions of a second in every cell of every creature on earth. Self-assembly is awe-inspiring; it allows form to emerge from the pieces and forces intrinsic to nature’s substances themselves.
How do those self-assembling proteins start moving things around in our bodies so our DNA instructions can be replicated? That will be the subject of the next essay in this series.


But are billions of years enough time?