In the previous essay we examined the complex life of birds. And in another previous essay, we explored the rules of quantum mechanics -- the physical laws that apply only at the scale of subatomic particles, and only when those particles are left undisturbed by interactions with the larger world that would destroy the internal “coherence” of the quantum system and subject it to the rules of classical physics that govern the macro-world we live in.
In this essay, we’ll explore the evidence, using JohnJoe McFadden and Jim Al-Khalili’s book Life on the Edge: The Coming of Age of Quantum Biology, that birds and other living things have capacities that could only be made possible through the use of quantum mechanical processes. It’s the fascinating world of quantum biology.
As McFadden and Al-Khalili write, robins and other birds and animals migrate to and from places halfway around the world using only their own biological compass attuned to the same Earth magnetic field that makes the frictionless needle on a handheld compass turn:
The mechanism that enables our robin to know how far to fly, and in which direction, is encoded in the DNA she inherited from her parents. This ability is a sophisticated and unusual one— a sixth sense that she uses to plot her course. For, like many other birds, and indeed insects and marine creatures, she has the ability to sense the earth’s weak magnetic field and to draw directional information from it by way of an inbuilt navigational sense, which in her case requires a novel type of chemical compass … Magnetoreception is an enigma. The problem is that the earth’s magnetic field is very weak—between 30 and 70 microtesla at the surface: sufficient to deflect a finely balanced and almost frictionless compass needle, but only about a hundredth the force of a typical fridge magnet. This presents a puzzle: for the earth’s magnetic field to be detected by an animal it must somehow influence a chemical reaction somewhere in the animal’s body—this is, after all, how all living creatures, ourselves included, sense any external signal. But the amount of energy supplied by the interaction of the earth’s magnetic field with the molecules within living cells is less than a billionth of the energy needed to break or make a chemical bond. How, then, can that magnetic field be perceptible to the robin? … The problem [a few decades ago] was that no one had a clue how any such biological inclination compass might work, because there was at that time simply no known, or even conceivable, mechanism that could account for how the angle of dip of the earth’s magnetic field could be detected within an animal’s body. The answer turned out to be within one of the most startling scientific theories of modern times, and it had to do with the strange science of quantum mechanics.
It turns out that, as Al-Khalili explains in this video here, it appears the robin’s eyes rely on the process of quantum entanglement at the deepest levels to trigger the chemical reactions that guide its path.
Also, as we explored in a previous essay, the science of quantum mechanics has shown that subatomic particles can operate like particles, but also like waves that move through a field, like ripples emanating from the center of a pond or a magnetic field radiating from the poles of a magnet. As it turns out, this wave behavior at the subatomic level helps explain why certain biological processes work, as they couldn’t work otherwise if subject to the classical physics governing both larger particles and smaller particles when influenced by the wider environment. This should probably come as no surprise. As McFadden and Al-Khalili write:
Biology is, after all, a kind of applied chemistry, and chemistry is a kind of applied physics. So isn’t everything, including us and other living creatures, just physics when you really get down to the fundamentals? … [S]ince the rules of quantum mechanics govern the behavior of atoms, and biology ultimately involves the interaction of atoms, then the rules of the quantum world must also operate at the tiniest scales within biology—but only at those scales.
But as we learned in a previous essay, quantum interactions are not governed by the laws of classical physics which can predict with certainty, for example, the trajectory of a cannonball. Instead, the behavior of things at the quantum level are subject only to probabilities that, as far as we know, are fundamental – that is, embedded in the system such that even with perfect knowledge of initial conditions and with wholly accurate calculations there are behaviors whose outcomes we could never predict with total confidence, but only with probabilities. The outcomes will always follow some probability distribution, which can be calculated. But as we also learned in a previous essay, when a quantum system interacts with its larger environment, the fundamental probabilities of the quantum mechanical system become “entangled” with the states of the environment. This interaction leads to the loss of coherence between the quantum mechanical components (“decoherence”), making the system appear classical and thus effectively collapsing the probabilities function and rendering it subject to the laws of classical physics, under which more concrete predictions can be made. But no one has been able to pinpoint exactly at which point a surrounding environment can “overwhelm” a quantum system and thereby cause it to behave under the rules of classical physics.
As McFadden and Al-Khalili write:
Why is there a fault line, an edge, between the world that we see and the world that physicists know really exists beneath its surface? This is one of the deepest problems in the whole of physics, and one that relates to the phenomenon of quantum measurement … When a quantum system interacts with a classical measuring device … it loses its quantum weirdness and behaves like a classical object. But the measurements carried out by physicists cannot be responsible for the way the world we see around us appears. So what is it that carries out the equivalent quantum-behavior-destroying function outside the physics laboratory? The answer has to do with the way particles are arranged and how they move within large (macroscopic) objects. Atoms and molecules tend to be randomly scattered and vibrating erratically inside inanimate solid objects; in liquids and gases they are also in a constant state of random motion due to heat. These randomizing factors—scattering, vibrations and motion—cause the wavy quantum properties of particles to dissipate very quickly. So it is the combined action of all the quantum constituents of a body that performs the “quantum measurement” on each and all of them, thereby making the world we see around us look normal.
But how can quantum rules govern within the wet, squishy, and busy environment inside a living cell? As McFadden and Al-Khalili explain:
Living cells were thought to be composed mostly of water and biomolecules in a constant state of molecular agitation that would be expected to instantly measure and scatter those weird quantum effects. By “measure” here we do not of course mean that water molecules or biomolecules perform a measurement in the sense that we might measure the weight or the temperature of an object and then make a permanent record of this value on paper or on a computer’s hard drive, or even only in our brain. What we are talking about here is what happens when a water molecule bumps into one of a pair of entangled particles: its subsequent motion will be affected by the state of that particle, so that if you were to study the water molecule’s subsequent motion you could deduce some of the properties of the particle it had bumped into. So, in this sense, the water molecule has carried out a “measurement” because its motion provides a record of the state of the entangled pair, whether or not anyone is there to examine it. This kind of accidental measurement is usually sufficient to destroy entangled states. So the claim that delicately arranged quantum entangled states could survive in the warm and complex interior of living cells was thought by many to be an outlandish idea, verging on madness.
Yet:
Quantum phenomena … have been detected in lots of biological phenomena, from the way plants capture sunlight to the way that all our cells make biomolecules … And one of the most exciting areas of research—the one that might have huge implications for the development of new quantum technologies—is the recent unraveling of the mystery of how quantum weirdness manages to survive in hot, wet and messy living bodies … The biggest question in science … is how the inert atoms and molecules found in rocks are transformed every day into running, jumping, flying, navigating, swimming, growing, loving, hating, lusting, fearing, thinking, laughing, crying, living stuff. Familiarity renders this extraordinary transformation unremarkable, but it is worth remembering that even in this age of genetic engineering and synthetic biology, nothing living has ever been made by humans entirely from nonliving materials … [R]ecent research show[s] that at least one of the missing pieces in the puzzle of life is found within the world of quantum mechanics, where objects can be in two places at once, possess spooky connections and travel through apparently impenetrable barriers. Life appears to have one foot in the classical world of everyday objects and the other planted in the strange and peculiar depths of the quantum world … But can animals, plants and microbes really be governed by laws of nature that we have thus far believed to describe only the behavior of fundamental particles? Surely living organisms made up of trillions of particles are macroscopic objects that, like footballs or cars or steam trains, should be adequately described by classical rules, such as Newton’s mechanical laws or the science of thermodynamics. To discover why we need the hidden world of quantum mechanics to account for the amazing properties of living matter, we need first to embark on a short tour of science’s efforts to understand what is so special about life.
As McFadden and Al-Khalili elaborate:
Moving objects … possess energy that could be transferred to stationary objects they bumped into, causing them to move. But forces could also be transmitted remotely between objects: examples of these were the gravitational force of the earth, which pulled Newton’s apple to the ground, or the magnetic forces that deflected compass needles … [But the] orderly motion of every heat engine that has ever been built is delivered by harnessing the average motion of trillions of randomly moving atoms and molecules. Not only that, but the science [of thermodynamics, explored in a previous essay] is extraordinarily general, applicable not only to heat engines, but to nearly all the standard chemistry that takes place whenever we burn coal in air, allow an iron nail to rust, cook a meal, manufacture steel, dissolve salt in water, boil a kettle or send a rocket to the moon. All these chemical processes involve the exchange of heat and they are, at a molecular level, all driven by thermodynamic principles that are based on random motion. In fact, almost all of the nonbiological (physical and chemical) processes that cause change in our world are driven by thermodynamic principles. Ocean currents, violent storms, the weathering of rocks, the burning of forests and the corrosion of metals are all controlled by the inexorable forces of chaos that underpin thermodynamics. Each complex process may appear structured and orderly to us, but at their core they are all driven by random molecular motion … [A] bird, a fish or a human … is able to sustain and replicate itself by harvesting free energy from random molecular collisions. And although this is a complex and difficult task, its driving force is generally considered to be exactly the same as that used for pushing steam trains up hillsides. In life, billiard balls are replaced by molecules obtained from food, but although the process is far more complex than that described in our simple example, the principle is the same: free energy harvested from random molecular collisions (and their chemical reactions) is directed to maintain a body and make a copy of that body [as was explored in a previous essay series on how DNA replicates with the help of enzymes and proteins] … These nanomachines of nature are performing, at a molecular level, a carefully choreographed dance whose actions have been precision engineered by millions of years of natural selection to manipulate the motion of the fundamental particles of matter.
Indeed, as McFadden and Al-Khalili write:
Nature long ago discovered a far more efficient means of capturing this energy, through the process of respiration. In fact, in terms of chemical complexity, respiration is probably second only to photosynthesis … To home in on the role that quantum mechanics plays here, we will need to simplify how respiration works. And even when simplified, it still involves a remarkable sequence of processes that beautifully convey the wonder of these biological nanomachines. It starts off with the burning of a carbon-based fuel, in this case the nutrients we get from our food. For example, carbohydrates are broken down in our gut to yield sugars, such as glucose, that are loaded into the bloodstream and then delivered to cells hungry for energy. The oxygen needed to burn this sugar fuel is delivered by the blood from the lungs to the same cells. Just as with the burning of coal, electrons in the outer orbits of carbon atoms within a molecule are transferred to a molecule called NADH. But instead of being used immediately to bond to the oxygen atoms, the electrons are passed from one enzyme to another along a respiratory chain of enzymes inside our cells, rather like the baton being passed from one runner to another in a relay race. At each transfer step the electron is dropped into a lower-energy state and the difference in energy is used to power enzymes that pump protons out of the mitochondria. The resulting proton gradient from the outside to the inside of the mitochondria is then used to drive the rotation of another enzyme, called ATPase, which makes a biomolecule called ATP. ATP is very important in all living cells as it acts as a kind of energy battery that can easily be transported around the cell to power lots of energy-hungry activities, such as moving or building bodies. The function of the electron-driven proton-pumping enzymes is a bit like that of hydroelectric pumps that store excess energy by pumping water up a hillside. The stored energy can then be released by letting the water flow down the hillside to rotate a turbine engine that generates electrical power. Similarly, respiratory enzymes pump protons out of the mitochondria. When the protons flow back inside, they power the rotations of the turbine-like ATPase enzyme. These rotations drive another set of choreographed molecular motions that bolt a high-energy chemical phosphate group onto a molecule within the enzyme to make ATP. Extending the analogy of this energy-capturing process as a relay race, we can imagine the baton being replaced by a bottle of water (representing the electron energy), with each runner (enzyme) taking a sip of water and then passing on the bottle, before finally the remainder of the water is poured into a bucket called oxygen. This capturing of the electron energy in small chunks makes the whole process much more efficient than simply pouring it directly into oxygen, as very little of it is lost as waste heat. So the key events of respiration actually have very little to do with the process of breathing, but consist instead of an orderly transfer of electrons through a relay of respiratory enzymes inside our cells.
And how does all this relate to quantum mechanics? As McFadden and Al-Khalili write:
Each electron transfer event, between one enzyme and the next in the relay, takes place across a gap of several tens of angstroms—a distance of many atoms—much farther than was thought to be possible for conventional electron-hopping. The puzzle of respiration is how these enzymes are able to shift the electrons so quickly and efficiently across such big molecular gaps … [Q]uantum tunneling is … a means by which particles can get from one side of a barrier to the other in a way that common sense tells us should be impossible. By “barrier” we mean here a physically impassable region of space (without sufficient energy)—think of force fields used in science fiction stories. This region could consist of a narrow insulating material separating two sides of electric conductors or even empty space, such as the gap between two enzymes in a respiratory chain … Consider the example of a ball being kicked up a small hill … According to classical Newtonian mechanics, the only way a ball can get across the barrier is for it to possess sufficient energy to be lifted over the energy hill. But if that ball were an electron, say, and the hill a repulsive energy barrier, then there would be a small probability that the electron would flow through the barrier as a wave, essentially making an alternative and more efficient passage through. This is quantum tunneling … A crucial feature of quantum tunneling is that, like many other quantum phenomena, it depends on the spread-out wave-like nature of matter particles. But for a body made up of very many particles to tunnel it has to maintain the wave aspects of all its constituents marching in step, with peaks and troughs of waves coinciding, something we refer to as the system being coherent, or simply “in tune.” Decoherence describes the process whereby all the many quantum waves very rapidly get out of step with one another and wash away any overall coherent behavior, thus destroying the body’s ability to quantum tunnel. For a particle to quantum tunnel, it must remain wavy in order to seep through the barrier. This is why big objects, such as footballs, do not quantum tunnel: they are made up of trillions of atoms that cannot behave in a coordinated coherent wave-like fashion. By quantum standards, living cells are also big objects, so at first glance it would seem unlikely that quantum tunneling would be found inside hot, wet living cells whose atoms and molecules would mostly be moving incoherently. But, as we have discovered, the interior of an enzyme is different: its particles are engaged in a choreographed dance rather than a chaotic rave. So let us explore how this choreography can make a difference to life … Few scientists now doubt that electrons travel along respiratory chains via quantum tunneling. This places the most important energy-harnessing reactions in animal and (nonphotosynthetic) microbial cells (we will be dealing with the photosynthetic sort in the next chapter) firmly within the sphere of quantum biology. But electrons are very light, even by the standards of the quantum world, and their behavior is inevitably very “wave-like.” They should not therefore be regarded as moving and bouncing about like tiny classical particles, despite the fact that they are still treated this way in many standard biochemistry texts that continue to use the “solar system” model of the atom. A much more appropriate representation of the electrons in an atom is as a spread-out, wavy cloud of “electronness” surrounding the tiny nucleus, the “cloud of probability” … It is perhaps not so surprising, therefore, that electron waves can pass through energy barriers rather like sound waves passing through walls … even in biological systems. But what about bigger particles, such as protons or even whole atoms? Can these also tunnel in biological systems? At first glance you would think the answer would be no. Even a single proton is two thousand times as heavy as an electron, and quantum tunneling is known to be exquisitely sensitive to how massive the tunneling particle is: small particles tunnel readily whereas heavy particles are far more resistant to tunneling unless the distances to be covered are very short. But recent remarkable experiments indicate that even these relatively massive particles are able to quantum tunnel in enzymatic reactions … Enzymes have made and unmade every single biomolecule inside every living cell that lives or has ever lived. Enzymes are as close as anything to the vital factors of life. So the discovery that some, and possibly all, enzymes work by promoting the dematerialization of particles from one point in space and their instantaneous materialization in another provides us with a novel insight into the mystery of life … [O]nly a decade or so ago that most scientists dismissed the idea that tunneling and other delicate quantum phenomena could be taking place in biology. The fact that they have been found in these habitats suggests that life takes special measures to capture advantages provided by the quantum world to make its cells work. But what measures? How does life keep that enemy of quantum behavior, decoherence, at bay? This is one of the biggest mysteries of quantum biology, but one that is slowly being unraveled … Life operates at high temperatures (by the standards of the quantum world). So, for most of the history of biochemistry, scientists assumed that enzymatic transfer of protons was mediated entirely by the (non-quantum) mechanism of hopping over the energy barrier [the minimum amount of energy that reactants must possess for a chemical reaction to occur].
But, again, we know that below the subatomic level things operate under the weird rules demonstrated by the “two-slit” experiment we explored in a previous essay:
What the two-slit experiment delivers is the simplest and starkest demonstration that, down in the quantum world, everything is different. Particles can behave like waves spread out across space and waves can sometimes act like individual localized particles … [W]ave–particle duality is also involved in the most important biochemical reaction in the biosphere: the conversion of air, water and light into plants, microbes and, indirectly, all the rest of us.
To briefly recap the results of the “two-slit” experiment wherein atoms are sent through slits made in a barrier:
The two-slit experiment doesn’t make (common) sense, but it is real and has been performed thousands of times … [I]nstead of individual atoms going through the two-slit experiment we have to consider the wave function traveling from source to back screen [a wave function is a mathematical function that provides information about the probability amplitude of a particle's position, momentum, or other physical properties]. On encountering the slits, the wave function splits in two, with each half going through one of the slits. Note that what we are describing here is the way an abstract mathematical quantity changes in time. It is pointless to ask what is really going on, since we would have to look to check. But as soon as we try to do so we alter the outcome. Asking what is really going on between observations is like asking whether your fridge light is on before you open the fridge door: you can never know because as soon as you peek you change the system. The question then arises: When does the wave function “become” a localized atom once again? The answer is: when we try to detect its location. When such a measurement takes place, the quantum wave function collapses to a single possibility … [Y]ou can think of each atom in your body as being observed, or measured, by all the other atoms around it, so that any delicate quantum properties it might have are very quickly destroyed … Returning to our analogy of throwing pebbles into water, when we threw them into a still pond it was easy to see their overlapping waves interfering with one another. But try throwing those same pebbles into the base of Niagara Falls. The hugely complex and chaotic nature of the water now immediately wipes out any interference pattern generated by the pebbles. This turbulent water is the classical equivalent of the random molecular motion surrounding a quantum system, resulting in instant decoherence. Most environments are, at a molecular level, just as turbulent as the waters at the base of Niagara Falls. Particles within materials are constantly being jostled and bumped around by their environment (other atoms, molecules or photons of light) … Quantum coherence is normally expected to be very short-lived unless the quantum system can be isolated from its surroundings (fewer jostling particles) and/or cooled to a very low temperature (much less jostling) to preserve the delicate coherence. In fact, to demonstrate interference patterns with single atoms, scientists pump all the air out of the apparatus and cool their equipment down to very close to absolute zero. Only by taking these extreme steps can they maintain their atoms in a quiet quantum coherent state for long enough to demonstrate the interference patterns. So the idea that quantum coherence could be maintained in the hot, wet and molecularly turbulent environment inside a blade of grass was understandably thought to be crazy.
McFadden and Al-Khalili begin to explain how quantum effects might occur in biological systems:
Glance up at the sky for one second and a column of light 186,000 miles long descends into your eye. In that same second, the earth’s plants and photosynthetic microbes harvest the solar light column to make about 16,000 tonnes of new organic matter in the form of trees, grass, seaweed, dandelions, giant redwoods and apples. Our aim in this section is to discover how this first step in the transformation of inanimate matter into nearly all of the biomass on our planet actually works; and our exemplar transformation will be the conversion of New England air into an apple on Newton’s tree.
McFadden and Al-Khalili invite us to imagine looking deep into the leaves of a plant:
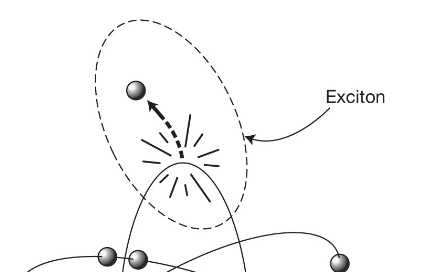
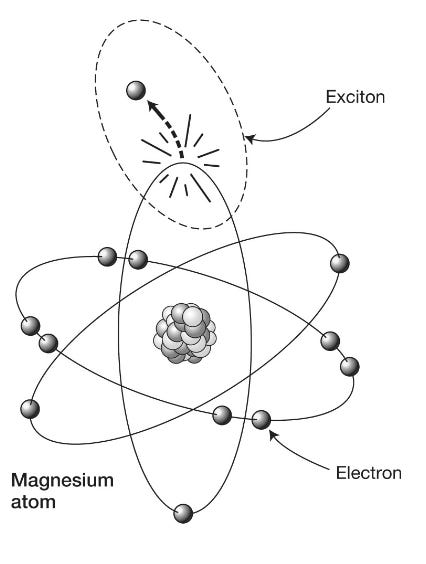
[T]rillions of photosynthetic machines that manufacture the world’s biomass. From your vantage point you can see that, as we discovered when examining enzyme machinery in the last chapter, although there are plenty of the billiard-ball-like turbulent molecular collisions going on all around you, there is also an impressive degree of order. The membranous surface of the thylakoid is studded with craggy green islands forested with tree-like structures terminating in antennae-like pentagonal plates. These antennae plates are light-harvesting molecules called chromophores, of which chlorophyll is the most famous example, and it is these that perform the first crucial step of photosynthesis: capturing light … Probably the second most important molecule on our planet (after DNA), chlorophyll is worth a closer look. It is a two- dimensional structure made up of pentagonal arrays of mostly carbon (gray spheres) and nitrogen (N) atoms enclosing a central magnesium atom (M), with a long tail of carbon, oxygen (O) and hydrogen (white) atoms. The magnesium atom’s outermost electron is only loosely bound to the rest of the atom and can be knocked into the surrounding carbon cage by absorption of a photon of solar energy to leave a gap in what is now a positively charged atom. This gap, or electron hole, can be thought of in a rather abstract way as a “thing” in itself: a positively charged hole. The idea is that we regard the rest of the magnesium atom as remaining neutral while we have created, through the absorption of the photon, a system consisting of the escaped negative electron and the positive hole it has left behind. This binary system is called an exciton (see figure 4.6) and can be thought of as a tiny battery with positive and negative poles capable of storing energy for later use.
Figure 4.6: An exciton consists of an electron that has been knocked out of its orbit in an atom, together with the hole it leaves behind.
Excitons are unstable. The electron and its hole feel an attractive electrostatic force pulling them together. If they recombine, the solar energy of the original photon is lost as waste heat. So, if the plant is to harness its captured solar energy, it has to transport the exciton very rapidly to a molecular manufacturing unit known as the reaction center, where a process called charge separation takes place. Essentially, this involves stripping an energetic electron completely from its atom and transferring it to a neighboring molecule … This process creates a more stable chemical battery (called NADPH) than an exciton that is used to drive the all-important photosynthetic chemical reactions. But reaction centers are usually quite distant, in molecular terms (nanometer distances) from the excited chlorophyll molecules, so the energy has to be transferred from one antenna molecule to another within the chlorophyll forest to reach the reaction center. This can happen thanks to the tightly packed nature of the chlorophyll. Molecules neighboring the one that has absorbed the photon can themselves become excited, effectively inheriting the energy of the initially excited electron, which is then transferred to their own magnesium atom’s electron. The problem, of course, is which route this energy transfer should take. If it heads in the wrong direction, randomly hopping from one molecule to the next in the chlorophyll forest, it will eventually lose its energy rather than delivering it to the reaction center. Which way should it turn? It doesn’t have very long to find its way to its destination before the exciton expires. Until recently, it was thought that this energy-hopping from one chlorophyll molecule to another was haphazard, essentially adopting the search strategy of last resort, known as a random walk. This is sometimes referred to as a “drunken walk” because it resembles the path taken by an intoxicated drinker exiting a bar, wandering this way and that until he eventually finds his way home. But random walks are not a very efficient means of getting anywhere: if the drunk’s home is far away, he may well wake up the following morning in a bush on the other side of town. An object engaged in a random walk will tend to move away from its starting point by a distance proportional to the square root of the time taken. If in one minute a drunk has advanced by one meter, then after four minutes he will have advanced by two meters and after nine minutes, only three meters. Given this sluggish progress, it is not surprising that animals and microbes seldom use a random walk to find food or prey, only resorting to the strategy if no other options are available. Drop an ant onto unfamiliar ground and as soon as it encounters a scent, it will abandon a random walk and follow its nose. Possessing neither nose nor navigation skills, the exciton energy was thought to advance through the chlorophyll forest via the drunkard’s strategy. But such a picture didn’t make much sense, as this first event in photosynthesis is known to be extraordinarily efficient. In fact, the transfer of captured photon energy from a chlorophyll antenna molecule to the reaction center boasts the highest efficiency of any known natural or artificial reaction: close to 100 percent. Under optimal conditions, nearly every energy parcel absorbed by a chlorophyll molecule makes it to the reaction center. If the path taken were a meandering one, nearly all of them, certainly most of them, should get lost. How this photosynthetic energy can find its way to its destination so much better than drunkards, ants or indeed our most energy-efficient technology has been one of the biggest puzzles in biology.
Researchers began to study exactly how fast photosynthesis was occurring:
To probe the chlorophyll sample, the researchers fired three successive pulses of laser light into the photosynthetic complexes. These pulses deposit their energy in very rapid and precisely timed bursts and generate a light signal from the sample that is picked up by detectors. Greg Engel [at Berkley], the lead author on the paper, spent the entire night stitching together the data generated from signals covering a time of fifty to six hundred femtoseconds, to produce a plot of their results. What he discovered was a rising and falling signal that oscillated for at least six hundred femtoseconds. These oscillations are akin to the interference pattern of light and dark fringes in the two-slit experiment; or the quantum equivalent of the pulsating sound beats heard when tuning a musical instrument. This “quantum beat” showed that the exciton wasn’t taking a single route through the chlorophyll maze but was instead following multiple routes simultaneously … The Berkeley group was suggesting that the [photosynthesis process] was acting as a quantum computer to find the quickest route to the reaction center, a challenging optimization problem … [T]he chlorophyll molecules were operating a novel search strategy known as a quantum walk … [T]he initial photon-capturing event responsible for putting most of the biomass on the planet appears to be dependent on a delicate quantum coherence that can be maintained for biologically relevant lengths of time within the warm but highly organized interior of a leaf or microbe … [L]iving cells do manage to keep decoherence at bay for long enough to transport excitons in photosynthetic complexes, or electrons and protons in enzymes.
How might that be possible? McFadden and Al-Khalili explain:
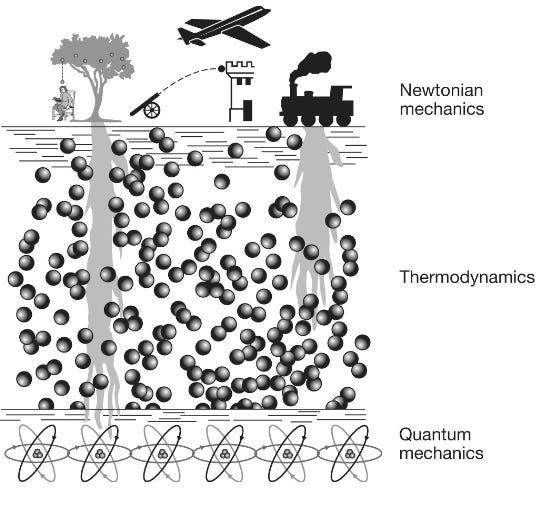
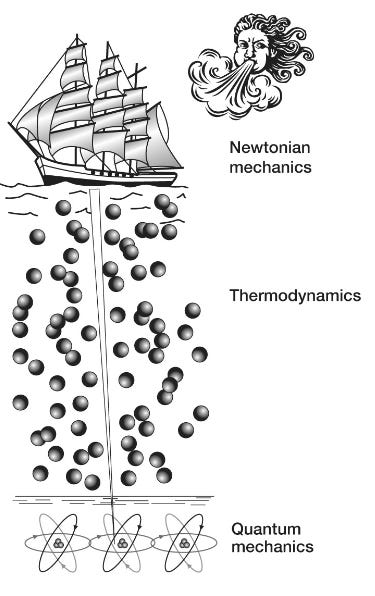
[W]e can view physical reality as consisting of three levels.
On the surface are the macroscopic, everyday objects such as footballs, trains and planets, whose overall behavior adheres to Newton’s mechanical laws of motion involving such familiar concepts as speed, acceleration, momentum and forces. The middle layer is the thermodynamic layer that describes the behavior of liquids and gases. Here, the same classical Newtonian rules apply; but, as Schrödinger pointed out … these underlying thermodynamic laws, which describe for example how a gas expands when heated or how a steam engine drives trains up hillsides, are based on the “order from disorder” averaging of the disorderly billiard-ball-like jostling of trillions of atoms and molecules. The third and deepest level is the bedrock of reality: the quantum world. Here is where the behavior of the atoms and molecules and the particles from which they are made obeys the precise and orderly rules of quantum, not classical, mechanics. However, most of the weird quantum stuff is generally invisible to us. It is only when we carefully observe individual molecules, as for example in the double-slit experiment, that we see the deeper, quantum laws. The behavior they describe appears unfamiliar to us because we normally see reality through a decoherence filter that strips out all the weirdness from bigger objects. Most living organisms are relatively large objects. Like trains, footballs and cannonballs, their overall motion adheres pretty well to Newtonian laws: a man fired out of a cannon has a similar trajectory as that of a cannonball. At a deeper level, the physiology of tissues and cells is also well described by the thermodynamic laws: the expansion and contraction of a lung is not so different from the expansion and contraction of a balloon. So at first glance you would tend to assume, and most scientists have assumed, that the quantum behavior similarly gets washed away in robins, fish, dinosaurs, apple trees, butterflies and us, just as it does in other classical objects. But we have seen that this is not always true for life; its roots reach down from the Newtonian surface through the turbulent thermodynamic waters to penetrate the quantum bedrock, allowing life to harness coherence, superposition (how quantum systems can exist in multiple states simultaneously until they are measured), tunneling or entanglement. The question we want to address in this final chapter is: How? … [M]any questions remain, principally concerning how life manages to maintain quantum coherence in the warm, wet sea of biomolecules within a living cell. Proteins or DNA are not steel-built machines with rigid parts, like the instruments used to detect quantum effects in physics laboratories; they are squishy, flexible structures that are constantly subjected to their own thermal vibrations as well as being continuously battered by the bumping of surrounding molecular billiard balls, a constant barrage of molecular noise. These random vibrations and collisions would be expected to shatter the delicate arrangement of atoms and molecules those particles need to maintain their quantum behavior. How this coherence is preserved in biology remains a puzzle; but, as we will discover, it is one that is beginning to be unraveled to reveal fascinating insights into how life works; insights that might even be exploited to drive the quantum technologies of the future … Some of the most exciting new results in this area are emerging from further studies of photosynthesis … [M]icrobes and plant leaves are packed full of chloroplasts filled with forests of chlorophyll pigment molecules, and that the first step in photosynthesis involves the capture of a photon of light by a pigment molecule and its conversion to an oscillating exciton that gets whisked through the chlorophyll forest to the reaction center. You will also remember that the signature of coherence, quantum beating, was detected in this energy transport process—evidence that its near 100 percent efficiency is thanks to excitons quantum walking their way to the reaction center. But how excitons maintain their coherent wave-like behavior while strolling through the molecularly noisy environment of a living cell has, until recently, been a puzzle. We have now discovered that the answer seems to be that living systems don’t try to avoid molecular vibration; instead, they dance to its beat … [Q]uantum coherence in photosynthesis [is] a kind of molecular version of an orchestra being “in tune” and “in time,” with all the coherent pigment molecules playing to the same beat. But the problem the system has to overcome is that the inside of the cell is very noisy. This molecular orchestra is playing not in a quiet concert hall, but in something more like a busy city center, amid a cacophony of molecular noise that disturbs each of the musicians so that their exciton oscillations are likely to be knocked out of tune, causing their delicate quantum coherence to be lost. This challenge is familiar to physicists and engineers attempting to build devices such as quantum computers [explored in a previous essay]. They tend to use two main strategies to keep the noise at bay. First, whenever they can, they cool their systems down to very close to absolute zero. At these very low temperatures, the molecular vibrations are damped, which in turn subdues the molecular noise. Second, they shield their equipment within the molecular equivalent of a sound studio, thereby keeping any environmental noise at bay. There are no sound studios inside living cells, and plants and microbes live in hot environments, so how do photosystems maintain their tuneful quantum coherence for so long? The answer appears to be that photosynthetic reaction centers exploit two varieties of molecular noise to maintain rather than destroy coherence. The first is a relatively weak and low-level noise, sometimes called white noise, which is rather like TV or radio static that is spread across all frequencies. This white noise comes from the thermal molecular jostling of all the surrounding molecules, such as water or metal ions, that are packed inside living cells. The second kind, sometimes called colored noise, is “louder” and limited to certain frequencies, just as colored (visible) light is limited to a narrow range of frequencies on the electromagnetic spectrum. The source of colored noise is the vibrations of the larger molecular structures within the chloroplasts, such as the pigment (chlorophyll) molecules and the protein scaffolds that hold them in place, which are composed of strings of amino-acid beads that are bent and twisted into shapes suitable for housing pigment molecules. Their bends and twists are flexible and they can vibrate, but they do so only at certain frequencies, rather like the strings of a guitar. The pigment molecules themselves also have their own vibrational frequencies. These vibrations generate the colored noise that, like a musical chord, is composed of just a few notes. Both white and colored noise appear to be exploited by photosynthetic reaction systems to help shepherd the coherent exciton to the reaction center … Let’s imagine that a leaf has just picked up a solar photon and converted its energy to an exciton. Considered classically, the exciton is a particle that is localized in space and time. But, as the double-slit experiment revealed, quantum particles also possess a diffuse wave character that enables them to exist in multiple places simultaneously as a quantum superposition. It is the exciton’s waviness that is essential for efficient quantum transport, for this enables it, like a water wave, to explore multiple paths simultaneously. But if its quantum waviness breaks on the molecularly noisy rocks of decoherence inside the leaf, then its waviness will be lost and it will become a localized particle stuck in a single position. The noise essentially acts as a kind of continuous measurement, and if it is very intense then decoherence will take place very quickly, before quantum coherence has a chance to help the exciton wave reach its destination … When the MIT team estimated the influence of molecular noise/vibrations in the bacterial photosynthetic complex, they discovered that quantum transport was optimal at temperatures around those at which microbes and plants perform photosynthesis. This perfect match between optimal transport efficiency and the kind of temperatures in which living organisms live is remarkable and, the team claim, suggests that three billion years of natural selection have fine-tuned the quantum-level evolutionary engineering of exciton transport to optimize the most important biochemical reaction in the biosphere. As they argue in a later paper, “natural selection tends to drive quantum systems to the degree of quantum coherence that is ‘just right’ for attaining maximum efficiency.” However, good molecular vibrations are not just limited to the white noise variety. “Colored” noise, generated by a limited set of vibrations of the chlorophyll molecules themselves, or even the surrounding proteins, is now also thought to play a key role in keeping decoherence at bay. If we imagine the white thermal noise as a molecular version of the static on a badly tuned radio, then the good vibrations of colored noise are akin to a simple beat like the Beach Boys’“bop bop” in their song “Good Vibrations.” But remember that the exciton also behaves in a wave-like manner to generate those coherent quantum beats that Graham Fleming’s group detected. Two recent papers from Martin Plenio’s group at the University of Ulm in Germany in 2012 and 2013 demonstrated that if the oscillation of the exciton and the oscillations of the surrounding proteins—the colored noise—are beating to the same drum then, when the coherent exciton gets knocked out of tune by the white noise, it can be knocked back into tune by the protein oscillations … Complexity theory studies the tendency of certain forms of random chaotic motion to generate order through the phenomenon of self-organization. For example … the molecules within liquids are moving entirely chaotically, yet when your bathtub is draining the water spontaneously flows around the drain in an orderly clockwise or counterclockwise direction. This macroscopic order can also be seen in the patterns of convection flow in a heated pot of water, in hurricanes, tornadoes, the red spot on Jupiter and many other natural phenomena … What is remarkable about all these systems is that the macroscopic order we can see is not reflected at the molecular level. If you had a very powerful microscope that could reveal the individual molecules that were flowing down your drain you might be surprised to see that their motions are nearly entirely random, with just a very slight bias from randomness in a clockwise or counterclockwise direction. At a molecular level, there is only chaos—but chaos with a slight bias that can generate order at a macroscopic level: order from chaos, as this principle is sometimes termed … Is there anywhere in the universe where we might expect to find this finely tuned degree of molecular order capable of exploiting delicate quantum effects in the subatomic world? … [There is] a curious fact regarding photosynthetic reaction centers. They are all equipped, not with a single chlorophyll molecule that might be able to operate a straightforward quantum heat engine, but with a pair of chlorophyll molecules known as a special pair. Although the chlorophyll molecules in the special pair are identical, they are embedded in different environments in the protein scaffold, which makes them vibrate at slightly different frequencies: they are slightly out of tune … [T]his structure provides photosynthetic reaction centers with the precise molecular architecture needed for them to work as quantum heat engines. [A quantum heat engine is a device that uses quantum mechanical systems to convert heat energy into work, much like a classical heat engine but operating on principles of quantum mechanics including wherein energy is absorbed or released in quantized amounts (quanta) during transitions between energy levels. Quantum effects can sometimes lead to efficiencies that approach theoretical limits more closely than classical engines.] The researchers showed that the chlorophyll’s special pair appears to be tuned to exploit quantum interference to inhibit inefficient wasteful energy routes and thereby deliver energy to the acceptor molecule with about 20 percent higher efficiency … [P]hotosynthetic reaction centers evolved between two and three billion years ago. So for nearly the entire history of our planet, plants and microbes seem to have been utilizing quantum-boosted heat engines—a process so complex and clever that we have yet to work out how to reproduce it artificially—to pump energy into carbon and thereby make all the biomass that formed microbes, plants, dinosaurs and, of course, us. Indeed, we are still harvesting ancient quantum energy in the form of fossil fuels that warm our homes and power our cars and drive most of today’s industry.
McFadden and Al-Khalili conclude with a final metaphor:
We have already taken on board his insight that life is a system dominated by order that goes all the way down, from highly organized whole organisms through the stormy thermodynamic ocean to the quantum bedrock below. And, crucially, these dynamics of life are delicately poised and balanced so that quantum-level events can make a difference to the macroscopic world … This macroscopic sensitivity to the quantum realm is unique to life and allows it potentially to exploit quantum-level phenomena, such as tunneling, coherence and entanglement, to make a difference to us all. But, and this is a big but, this exploitation of the quantum world can only take place if decoherence can be kept at bay … Scientists have fended off decoherence by shielding their quantum reactions from intrusive “noise.” … [L]ife appears to have adopted a very different strategy. Instead of allowing noise to hinder coherence, life uses noise to maintain its connection to the quantum realm … [W]e will make a metaphorical shift by [using] a tall sailing ship. Our imaginary sailing ship will initially be in dry dock, with its narrow keel exquisitely balanced on a single line of carefully aligned atoms. In this perilously poised state our ship, like a living cell, is sensitive to quantum-level events taking place in its atomic keel. The tunneling of a proton, the excitation of an electron or the entanglement of an atom can all have an influence on the entire ship, perhaps by affecting its delicate balance on the dry dock. However, we will further imagine that its captain has found clever and surprising ways to make good use of these delicate quantum phenomena such as coherence, tunneling, superposition or entanglement to help navigate his craft once it sets sail. But remember that we are still in dry dock: this ship isn’t going anywhere just yet. And although in its delicately balanced state it can potentially harness quantum-level phenomena, its precarious perch leaves it vulnerable to even the faintest imaginable breeze—perhaps being touched by just a single air molecule—which could topple the whole vessel. The engineer’s approach to the problem of keeping the craft upright and thus retaining its sensitivity to the quantum events in its keel would be to enclose the ship in a shielded box and pump out all the air to prevent any stray billiard-ball-like molecule from disturbing the vessel. The engineer would also cool the entire system down to close to absolute zero so that not even a molecular vibration could disturb its delicate balance. But skilled sea captains know that there is another way to keep a ship upright: it must first be launched into turbulent thermodynamic waters. We take it for granted that a ship is easier to keep upright in water than on land, but thinking about it at a molecular level we find that the reason for its increased stability isn’t immediately obvious. We have just said that an engineer’s approach to keeping a narrow-keeled ship upright in dry dock would be to protect the vessel from any potential disturbance from stray atoms or molecules. But isn’t the sea full of stray atoms and molecules randomly jostling one another and the keel of any ship in that billiard-ball fashion? How is it that the precariously balanced ship can be toppled by tiny impacts on land but remains impervious to them when on the water? The answer comes back to those “order from disorder” rules that Schrödinger described. The ship will indeed be bombarded with trillions of molecular impacts from both its port and starboard sides. Of course, it is now no longer balancing on its ultrathin keel but kept afloat by the buoyancy of the water, and with so many impacts on both sides of the ship, the average force to the bow and stern or to port and starboard will be the same. So buoyant ships do not topple because they are being held up by trillions of random molecular bombardments: order (the ship’s vertical orientation) from disorder (trillions of random billiard-ball-like molecular impacts). But ships can of course be toppled, even on the high seas. Imagine that the captain has launched his ship onto a tempestuous sea, but hasn’t yet hoisted its sail. The waves buffeting the vessel aren’t so random anymore, and big swells may surge from one side or another that could easily topple an unstable vessel. But our clever captain knows how to increase the stability of his ship: he hoists the sails so that he can harness the power of the wind to keep his vessel on an even keel. Once again, this stratagem may, at first sight, appear to be contradictory. We would expect that haphazard winds and unpredictable gusts would act to topple rather than stabilize an already unsteady ship—particularly as they won’t be random but will tend to arrive with more force on one side of the ship or the other. But the captain knows how to adjust the angle of the sail and the tiller so that the action of the wind and currents acts against the gusts and the gales to correct any listing to one side or the other. In this way, he can harness the surrounding tempest to keep his ship stable. Life, it seems, is like that metaphorical ship sailing through stormy classical waters with a clever captain on board: the genetic program, honed by nearly four billion years of evolution, is able to navigate the various depths of the quantum and classical realms. Rather than hiding from the tempests, life embraces them, marshaling their molecular squalls and gales to fill its sails and keep the ship upright so that its narrow keel penetrates the thermodynamic waters to connect with the quantum world. Life’s deep roots allow it to harness those weird phenomena that prowl the quantum edge.
That concludes this essay on the possibility of quantum biology, a field that seems ripe for future exploration. And if you’re interested, Al-Khalili explains the possibility of quantum biology in this TED Talk lecture here.


Paul, You actually did it -- I thought you might be kidding. You merged the birds and quantum theory. This is a head spinning article. I will have to read it a couple more times to get it all down. I can count on learning something every time you publish something...and that's amazing. Many thanks.
Does this mean that if the Many Worlds theory is correct, there are worlds where all biological processes suddenly stop working because the particles in that world moved differently? Or are the quantum entanglement and decoherence processes being discussed in this article not the same processes that the Many Worlds theory explains?