Our Bodies’ Amazing Electric Current – Part 2
How our protein battery ion channels work, and the fascinating example of the electric eel.
Continuing our essay series on our bodies’ electric current, primarily through Frances Ashcroft’s The Spark of Life: Electricity in the Human Body, and Sally Adee’s We Are Electric: Inside the 200-Year Hunt for Our Bodies’ Electric Code, and What the Future Holds, this essay explores how our protein battery ion channels work, and the fascinating example of the electric eel. It’s another example of how evolution resulted in yet another astounding set of biological mechanisms.
As Ashcroft writes:
How many kinds of [ion] channel[s] are there? What do they do? And how exactly do they work – what kind of molecular gymnastics do they perform when they open and shut, and how do they pick and choose which ions they let through? Proteins are formed from a linear string of amino acids but – like a bead necklace dropped on the floor – they fold up into far more complex shapes. Some of the protein may become embedded in the membrane while other bits sit inside or outside of the cell. The protein may even twist around so that part of its structure becomes inverted … It turns out that because similar charges repel one another and opposite charges attract, many channels use rings of charge at their entrances to exclude or enhance ion entry. For example, by using negative charges – which will encourage cations to enter, but repel anions [note: negatively charged ions are called anions and positively charged ions are called cations] – a channel can permit the passage of all positively charged cations, but exclude negatively charged anions. The crucial problem most ion channels must solve, however, is how to produce high selectivity without slowing down the rate at which ions move through the pore. And one of the most difficult questions to answer was how potassium channels allow potassium ions to permeate but not the much smaller sodium ions, which are also positively charged. It mystified scientists for years. The X-ray structure showed in dazzling detail how the potassium channel works and how it is able to support a very rapid throughput of potassium ions, so fast it seems there is no barrier to ion movement at all, while at the same time excluding the smaller sodium ions. Potassium channels, it turns out, have evolved specialized ‘selectivity filters’, short regions where the pore narrows so much that permeating ions must interact with its walls. Simply put, this region is just wide enough for a potassium ion to squeeze through, but nothing larger can pass. The passage is so small, in fact, that potassium has to shed its coat of water molecules to squeeze through. In solution, all ions are clothed in bulky coats of water and it takes a lot of effort to remove them. Potassium is happy to shrug off its coat because the selectivity filter mimics the embrace of its watery jacket. Not so, sodium. Although sodium is small enough to slip through the pore when dehydrated, the effort required to strip off its water shell is too much – much greater than the energy supplied by the clinch of the selectivity filter – so it remains fully clothed. And with its coat on, sodium is simply too big to enter.
As Adee adds:
I like to think of ion channels as shape sorters—you know, the toy you give a baby so it can shove different-shaped pegs into a wooden box through matching holes. Some of the pegs are round, others are triangular, or squares, or stars. The square holes accommodate the square pegs, and so on. So, while some holes may be technically bigger than their non-matching counterparts, they still won’t fit through. They are incompatible with the dimensions of the channel, and therefore it remains impenetrable. (It’s actually a little more complicated still, because the holes in this baby toy shape-shift to accommodate the peg they like best.)
How do nerve cells communicate information? As Ashcroft writes:
Nerve cells talk to one another by means of chemical messengers, known as transmitters, which interact with specialized ion channels in the membrane of the target cell. The transmitter binds to a specific site on the channel protein, fitting snugly into its receptor like a key in a lock. When it does so, it triggers a conformational change in the channel protein that opens the pore and enables ion flow. We still know little about how such shape shifting takes place, or how binding of a chemical at one site leads to a structural change in another part of the protein, which may be far distant. But this type of gating is very important, not just for transmitting information between cells but also because many medicinal drugs and many poisons influence channel activity (and thereby cellular functions) by binding to the same site as the native transmitter and either blocking, or mimicking, its action. ‘Voltage-dependent’ gating requires that the channel is able to sense a change in the voltage field across the membrane. All cells have a potential difference across their membranes, the inside of the cell being about 70 millivolts more negative than the outside. When a nerve fires an electrical impulse this potential suddenly alters by about 100 millivolts, the inside of the cell briefly becoming positive with respect to the outside. How the channel senses the voltage field has only been discovered in the last twenty-five years and the precise details are still the subject of heated debate. In resting nerve and muscle cells, the voltage-gated sodium and potassium channels are held firmly shut by the negative membrane potential. They open only when the membrane potential becomes more positive and when this happens it triggers an electrical impulse. How this is achieved and the long and intricate work needed to unravel the story of how nerves and muscles work is considered in the next few chapters. Nerve fibres are used to transmit electrical signals around the body. What we generally refer to as a nerve is in fact a collection of many nerve fibres bundled together within a protective outer sheath, rather like a cable containing thousands of different telephone wires … Nerve cells are the building blocks of the nervous system, including the brain. They come in many shapes and sizes, but all consist of a cell body from which extend a number of fine, branched processes. Usually one of these processes is much longer than the others and is known as the nerve fibre or axon. It can be extremely long. The axons in your ulnar nerve, for example, run from your spinal cord to your fingers. The vagus nerve – the longest of the cranial nerves – runs from the brain to the stomach … Yet despite its length, a single nerve fibre is very thin, with a diameter less than a tenth of that of a human hair. Although nerve fibres are capable of conducting impulses in either direction, they usually only transmit them in one direction. Motor nerves conduct signals outwards from the brain and spinal cord to direct muscle contraction, whereas sensory nerves conduct information in the opposite direction, from our sense organs to the brain.
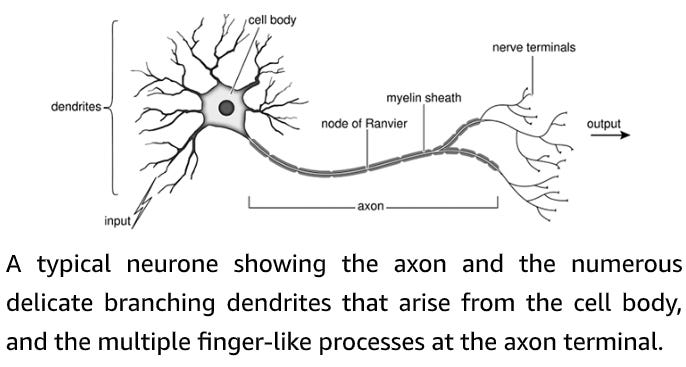
The cell body is the control centre of the nerve cell: it houses the nucleus where the genetic material (the DNA) is stored. Multiple short processes branch off the nerve cell body like the limbs of a tree, hence they are named dendrites, from the Greek dendron, meaning ‘tree’. The dendrites receive numerous signals from other nerve cells and serve as first-line information processing centres, integrating all incoming information before passing it on to the cell body. Nerve cell bodies lie almost exclusively within the brain and spinal cord, where they are protected by a ‘blood–brain barrier’ which separates the blood from the cerebrospinal fluid bathing the brain and spinal cord. The brain serves as the command centre of the entire nervous system. It contains millions of nerve cells, each of which has multiple processes and multitudinous connections to other brain cells. Nerve cells transmit information by means of electrical signals known as nerve impulses or action potentials. These race along the nerve fibre at speeds of up to 400 kilometres per hour (250 miles per hour). The fastest nerves of all are those that are enveloped in an insulating myelin sheath. This is formed from layer upon layer of membranes of a specialized cell (the Schwann cell) that wraps itself tightly around the axon like the layers of a Swiss roll, or the paper layers enveloping a toilet roll tube. This insulating myelin sheath enables nerve fibres to conduct electrical impulses more rapidly. When it is damaged, nerve conduction is disrupted. A myelinated nerve, showing the layers of insulating myelin wrapped around the nerve axon. The small organelle in the centre of the nerve is a mitochondrion, one of the cell’s power plants. By the middle of the last century it was appreciated that nerves and muscles transmit information in the form of electrical impulses, but exactly how the nerve impulse was generated and propagated along the nerve fibre was still a mystery. The pioneering experiments that led to the solution of this problem were carried out using single nerve fibres of the squid, giving this animal a special place in the hearts of physiologists … [Alan[ Hodgkin and [Andrew] Huxley’s elegant experiments revealed precisely how the nerve generates an electrical impulse. The action potential is caused by an initial increase in the permeability of the membrane to sodium ions. This is produced by the opening of sodium channels, which allows positively charged sodium ions to rush into the nerve cell and drive the membrane potential positive (depolarization). Less than a millisecond later, the potassium channels open, permitting potassium ions to exit the nerve and return the membrane potential to its resting level (repolarization). Together, these opposing ion fluxes generate a transient change in voltage that constitutes the nerve impulse. I vividly recall from the time I spent at Woods Hole that the axons which generated the best results were commemorated in a most singular fashion. At the end of the experiment, they were flicked onto the ceiling of the laboratory, which eventually acquired a pattern of dried-out squiggles, somewhat reminiscent of a Jackson Pollock painting. Only the very best axons, however, were ‘sent to Heaven’. Sodium and potassium channels that open in response to changes in the voltage gradient across the cell membrane are the keystone of electrical signalling in our brain, heart and muscle. In resting nerve cells, both kinds of channel are tightly shut. When the nerve is stimulated, first the sodium channels and then, with a short delay, the potassium channels swing into action producing a transient change in membrane potential – the nerve impulse. But what triggers the whole thing off? Crucially, the sodium and potassium channels that are involved in the action potential are sensitive to voltage and they open if the membrane potential is made more positive (depolarized). This is exactly what happens when a nerve cell is excited by an incoming signal from another nerve cell, or by an externally applied electric shock.
Communication within our bodies also occurs across small gaps:
Transmission of information from nerve cell to muscle cell (or another nerve cell) takes place at specialized junctions called synapses, where the gap between the two cells is very tiny – less than one hundred-millionth of a metre (about thirty nanometres). For obvious reasons, the upstream cell that releases the transmitter (in this case the nerve cell) is known as the pre-synaptic cell and its target as the post-synaptic cell. The tip of the nerve fibre is densely packed with small membrane-bound vesicles filled with a chemical transmitter. At the synapse between nerve and muscle the transmitter is acetylcholine, but many other chemicals are used to signal information between different types of nerve cells in the brain. When an electrical impulse arrives at the nerve ending it causes the vesicles to release their contents into the gap between the two cells. The transmitter that is liberated diffuses across the gap and attaches to a receptor on the surface of the post-synaptic cell, triggering an electrical impulse. In a muscle cell this electrical impulse causes contraction. When a nerve impulse arrives at the nerve terminal, it causes calcium channels to open, allowing calcium ions to flood into the cell. This triggers synaptic vesicles filled with the neurotransmitter acetylcholine to move to, and fuse with, the cell membrane, releasing their contents into the synaptic gap. Acetylcholine then diffuses across the gap and binds to its receptors in the muscle fibre membrane. Binding of the neurotransmitter opens an intrinsic ion channel in the acetylcholine receptor, enabling sodium ions to enter the cell. The flow of sodium current triggers an electrical impulse in the muscle. In this way, the electrical signal passes from nerve to muscle via a chemical intermediary.
Ashcroft then discusses muscle fibers:
Like a nerve fibre, and indeed all other cells in your body, muscle fibres have a voltage difference across their membranes, with the inside of the cell being more negative than the outside. Opening of the acetylcholine receptor channels dissipates this voltage difference, driving the membrane potential positive. Just as we saw with nerve cells, the change in membrane voltage opens muscle sodium channels and so sets off an electrical impulse (an action potential) that propagates along the muscle fibre in both directions from its point of origin. The action potential spreads rapidly over the surface of the muscle cell and then down into a network of tubular invaginations of the surface membrane, which penetrate right into the centre of the fibre. These conduct the action potential deep into the fibre interior, thus ensuring that all the contractile filaments contract in a single concerted step … The membranes of our muscles are full of ion channels. Calcium channels in the tubular membranes sense the voltage difference across the surface and tubular membranes and pass this information onto the ryanodine receptors, which sit in the intracellular membranes of the sarcoplasmic reticulum, the muscle’s calcium store. When the ryanodine receptors open, calcium rushes out, binds to the contractile filaments and causes the muscle to shorten.
Ashcroft then discusses the amazing comparisons between evolution-made electric eels and human-made batteries:
Electric eels have no teeth and must swallow their prey in one gulp, which is obviously harder if it is wriggling and may be why they generate electric shocks to stun their prey. Much of the time they lurk in the mud on the river bottom, but as they get most of their oxygen by gulping air they must surface every few minutes or so to breathe. Because they breathe air they do not die if they are removed from the water and so can be easily studied.
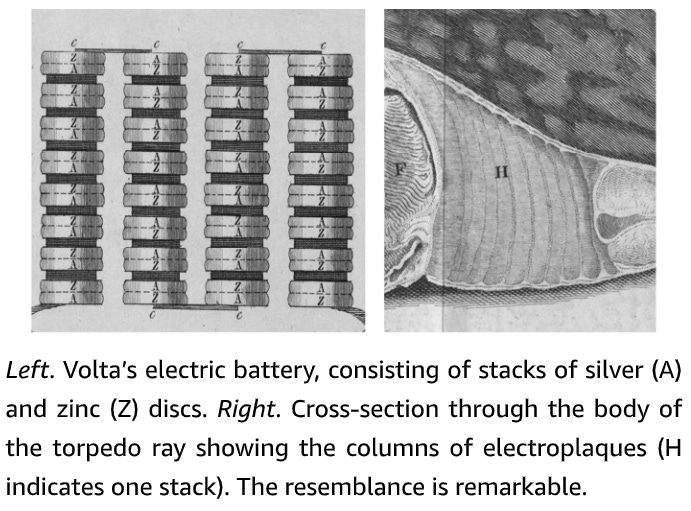
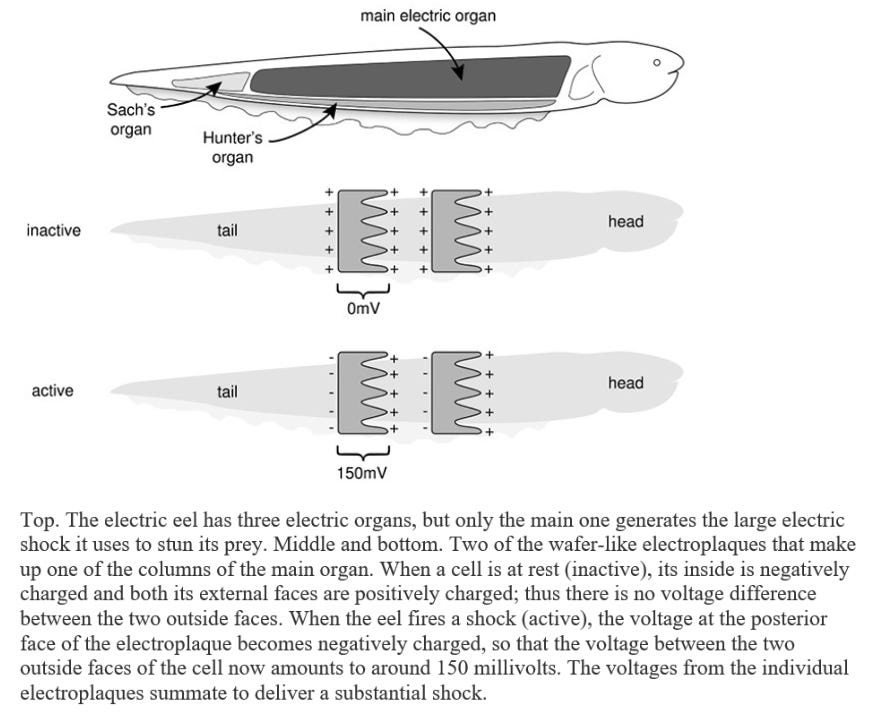
Electrophorus has a long, cylindrical, eel-like body, with a dark-grey back and yellowish belly, and it can reach an enormous size. Larger specimens weigh over twenty kilograms, exceed two and a half metres in length and are as thick as a man’s thigh. The vital organs are crammed into the front one-fifth of the body: the rest of the fish houses the backbone and swimming muscles, but most is pure power pack. The main electric organs lie on either side of the eel’s body. Each contains thousands of modified muscle cells, known as electroplaques, which have lost the capacity to contract and are specialized for producing an electric discharge. These wafer-thin, plate-like cells are stacked up in long columns, like a giant pile of coins, with as many as 5,000 to 10,000 cells per column. There are around seventy such columns on each side of the eel’s body. Each stack of electroplaques bears a strong similarity to a voltaic pile – the primitive battery discussed in Chapter 1 – a fact which Volta himself noted. The two faces of the electroplaque cell are markedly different. One side is smooth and criss-crossed by many nerve endings: the other is deeply invaginated and is not innervated. At rest there is no difference in voltage between the two outer faces of the cell and thus no shock is produced. When the fish decides to zap its prey, it fires off an impulse down the nerve supplying the electric organ. This triggers an electrical impulse in the electroplaque – in effect a muscle action potential – that is confined to the innervated side. As a consequence, a voltage difference develops across the two sides of the cell of as much as 150 millivolts. Because this happens simultaneously in all electroplaques, and because they are arranged in series, the voltages add up to produce a considerable jolt of 500 volts or more (about four times as much as a household electrical socket in the USA and twice as much as one in Europe). Thousands of muscle action potentials, all firing in synchrony, thus underlie the shock. In essence, each electroplaque behaves like a miniature living battery with the stimulated side (facing the tail) bearing a negative charge and the opposite side (facing the head) a positive charge. These tiny batteries are stacked up in a head-to-tail fashion in a long column.
Recharging the electric organ takes some time and is achieved by molecular pumps that laboriously pump all the sodium ions that have entered the cell back out again, thereby restoring the sodium gradient that powers the electrical impulse … It is also believed that fatty layers in the fish’s skin act as an insulator to protect it from its own shocks, because if the skin is scratched or damaged (which renders this insulation less effective) an electric eel twitches when it discharges, suggesting it now feels the shock. Of course, it is also important that the skin above the electric organs is not well insulated so that the current can escape into the water and, as expected, the skin over the top and bottom of the torpedo’s electric organs is of higher conductance than that covering other areas of the body.
In the next essay in this series, we’ll examine the methods of communication used by the heart, and our senses.