In this series of essays, we’ll examine one of the two main modes of communication within our bodies. Those modes are electric current and hormones. In this series of essays, we’ll focus on our bodies’ electric current, and its amazing capacities honed by evolution, primarily through Frances Ashcroft’s The Spark of Life: Electricity in the Human Body, and Sally Adee’s We Are Electric: Inside the 200-Year Hunt for Our Bodies’ Electric Code, and What the Future Holds.
As Ashcroft writes in The Spark of Life:
We’re all familiar with the fact that machines are powered by electricity, but it’s perhaps not so widely appreciated that the same is true of ourselves. Your ability to read and understand this page, to see and hear, to think and speak, to move your arms and legs – even your sense of self – is due to the electrical events taking place in the nerve cells in your brain and the muscle cells in your limbs. And that electrical activity is initiated and regulated by your ion channels. These little-known but crucially important proteins are found in every cell of our body and in those of every organism on Earth, and they regulate our lives from the moment of conception until we draw our last breath. Ion channels are truly the ‘spark of life’ for they govern every aspect of our behaviour. From the lashing of the sperm’s tail to sexual attraction, the beating of our hearts, the craving for yet another chocolate, and the feel of the sun on your skin – everything is underpinned by ion channel activity.
As Adee adds:
We are fundamentally electrical creatures, but the full extent of our electrification would shock you. It is hard to overstate how wholly and utterly your every movement, perception, and thought are controlled by electrical signals. This is not the electricity that comes from a battery or the kind that turns on the lights and powers the dishwasher. That kind of electricity is made of electrons, which are negatively charged particles flowing in a current. The human body runs on a very different version: “bioelectricity.” Instead of electrons, these currents are created by the movements of mostly positively charged ions like potassium, sodium, and calcium. This is how all signals travel within the brain and between it and every organ in the body via the nervous system, enabling perception, motion, and cognition. It’s fundamental to our ability to think and talk and walk and why our knee hurts after a fall, and why the scraped skin heals. It’s what makes gummy bears taste sour, why we can pick up a glass of water to wash away the taste, and how we know we were thirsty in the first place. The stuff that comes out of your wall socket is created by a power plant. For the stuff in your body, the power plant is you. Every one of the 40 trillion cells in your body is its own little battery with its own little voltage: when it’s at rest, the inside of a cell is (on average) around 70 millivolts more negatively charged than the extracellular soup outside. To keep it that way, the cell is constantly shuttling ions in and out of the membrane that surrounds it, always striving to maintain that -70mv.
Ion channels can act similarly to batteries in certain ways. Both ion channels and batteries involve the movement of ions, which generates an electric current. Ion channels rely on ion gradients across cell membranes, where there is a difference in ion concentration between the inside and outside of the cell. This gradient creates a potential difference, similar to how a battery has a potential difference between its terminals. When an ion channel opens, ions move through it, driven by the electrochemical gradient. This movement of ions constitutes an electric current, much like the flow of electrons in a circuit powered by a battery. In cells, the energy for maintaining ion gradients often comes from ATP [adenosine triphosphate, a nucleotide that provides energy to drive and support many processes in living cells], while in a battery, chemical reactions within the battery generate the potential difference and current. There are, of course, significant differences between ion channels and batteries as well. Ion channels are typically highly selective and can be gated, meaning they open or close in response to specific signals, whereas a battery continuously provides a steady voltage until it is depleted.
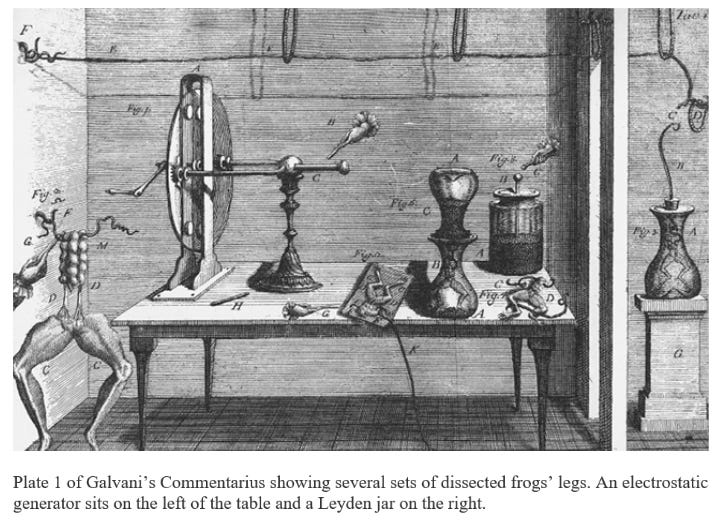
Ashcroft begins the scientific story with the Italian scientist Luigi Galvani, who studied the movement of electricity through frogs’ legs:
Being a careful scientist, Galvani repeated [his] experiment [using frogs legs] on a calm day, as a control. This time he suspended the frog’s legs from the iron railings of his balcony by brass hooks that pierced the spinal cord. At first, nothing happened. Getting impatient, Galvani began to fiddle with the frog’s legs. To his surprise, he noticed they then began to display frequent spontaneous and irregular movements, none of which depended on the variations of the weather, but which occurred when the hooks were pressed against the railing. Galvani interpreted this result to indicate that animal cells are not only stimulated by electricity – they can actually produce their own. This electrical (self)-stimulation, he surmised, produced contraction of the muscle … Galvani had a few copies of his article published at his own expense and sent them to his fellow scientists, including his friend and countryman, Alessandro Volta, who was professor of physics at the University of Pavia.
Ashcroft then describes an interesting series of events, in which Galvani’s experiments, in a roundabout way, inspired Volta to invent the electric battery:
Volta’s experiments caused him to revise his initial conclusion that Galvani was correct, and argue instead that the muscle twitches Galvani had observed in the absence of extrinsic electrical stimulation were not due to an innate animal electricity. Rather, he deduced (correctly) that they were induced by an electric current flowing between two dissimilar metals – the iron railing of the balcony and the brass hooks which Galvani had attached to the nerve supplying the frog’s leg … Volta … continued to explore the idea that contact between dissimilar metals was involved. Believing that electricity was not of animal origin, he decided to dispense with the frog altogether. He built a stack of alternating silver and zinc discs, separated by cardboard soaked in salt water, and demonstrated that an electric current flowed when the top and bottom of the pile were connected. He had invented the first electric battery. Indeed, he got an electric shock by touching one hand to the top and the other to the bottom of the pile.
Ashcroft then discusses exactly what electricity is, and what ion channels are:
[I]t is necessary to understand what ion channels are and how they contribute to the electrical responses in cells … [Let’s] therefore jump straight to the present day and provide a state-of-the-art picture of how ion channels work. But first it is helpful to consider what electricity is and how the electricity in your head differs from that supplied to your home. Electricity is a form of energy that is based on electric charge, one of the most fundamental properties of subatomic matter. The electric currents that flow through the wires in our houses – and along our nerves – are quantified in terms of three basic units: the amp (A), the volt (V), and the ohm (Ω). Current is measured in amps, resistance to current flow in ohms, and voltage, the force that drives the current flow, in volts. The flow of electric current through a wire is often explained by analogy with the flow of water through a pipe. In electrical terms, the current corresponds to the rate at which a stream of charged particles moves, with one amp being the equivalent of approximately six million million million (6x1018) particles per second. Resistance is a measure of the ease or difficulty of flow. Narrowing a pipe will restrict the flow of water, whereas increasing the pipe’s diameter will increase water flow. In an electric circuit, materials that offer little resistance to current flow, such as metals, are called conductors, whereas those that resist the flow of current (like paper or air) are known as insulators. Grasp the bare wire of an electric fence and you will get an unpleasant shock, but you will feel nothing if you instead take hold of one of the insulated handles that enables you to open the gate in the fence. The voltage difference between one point and another is analogous to the difference in water pressure that causes water to flow from one region to another. Essentially it is the force that drives the current flow. It is also sometimes called the electrical potential difference (or potential for short). Providing two points are not connected, no water will flow between them, and in an analogous way an electric current will only flow when a circuit is complete. This is why there can be a huge voltage difference between a thundercloud and the ground, but no current flows until the lightning bolt jumps the gap. It also explains why electrons do not move along a wire unless the electric circuit is complete, and thus why your desk lamp does not light up until you flick the on/off switch that links the wires. Just as increasing the water pressure will increase the flow of water, so boosting the voltage increases the current. Increasing the voltage supplied to the lamp, for example, will make it shine brighter … Ground (or earth) is defined as the lowest point of voltage and, like water, current will always flow to the lowest level … This is also the reason you get an electric shock when you accidentally touch a live wire: the ground on which you stand is at a lower voltage than the wire in your hand, so that the current flows through you to the ground. Amps, volts and ohms are bound together in an eternal embrace, as was first appreciated by Ohm. He formulated a famous law, which states that the current (I) is equal to the potential (V) divided by the resistance (R); it is abbreviated mathematically as I=V/R. In other words, provided that the resistance remains the same, increasing the voltage will increase the magnitude of the current that flows. Similarly, if the resistance falls, but the voltage remains the same, then the current will increase. And so on. This simple formula, known as Ohm’s Law, is the key to understanding how nerves – and electricity – work.
But our ion channels don’t work the same way our household appliances work. As Ashcroft explains:
There is a fundamental difference, however, between the electricity that powers our bodies and that which lights our cities. The electricity supplied to our homes is carried by electrons. These indivisible subatomic particles carry a negative electric charge and because opposite charges attract one another (and similar charges repel) electrons always flow from a region of negative to positive charge. Confusingly, we define current as the direction of flow of positive charges, which means that the current in a wire moves in the opposite direction to that in which the electrons flow! … In contrast, almost all currents in the animal kingdom are carried by ions – electrically charged atoms. There are five main ions that carry currents in our bodies. Four are positively charged – sodium, potassium, calcium and hydrogen (protons) – and one, chloride, is negatively charged. Because they are electrically charged, the movement of ions creates an electric current. In the case of positively charged ions, the current flow is in the same direction as the flow of ions, whereas for negatively charged ions (as for electrons) it is in the opposite direction. It is also worth noting that currents in electric circuits flow along the length of the wire. In contrast, the ion currents responsible for nerve impulses flow across the membranes that envelop our cells, into or out of the cell. Thus although electrical impulses travel along the length of our nerve and muscle fibres, the ion currents that generate them flow at right angles to the direction of travel.
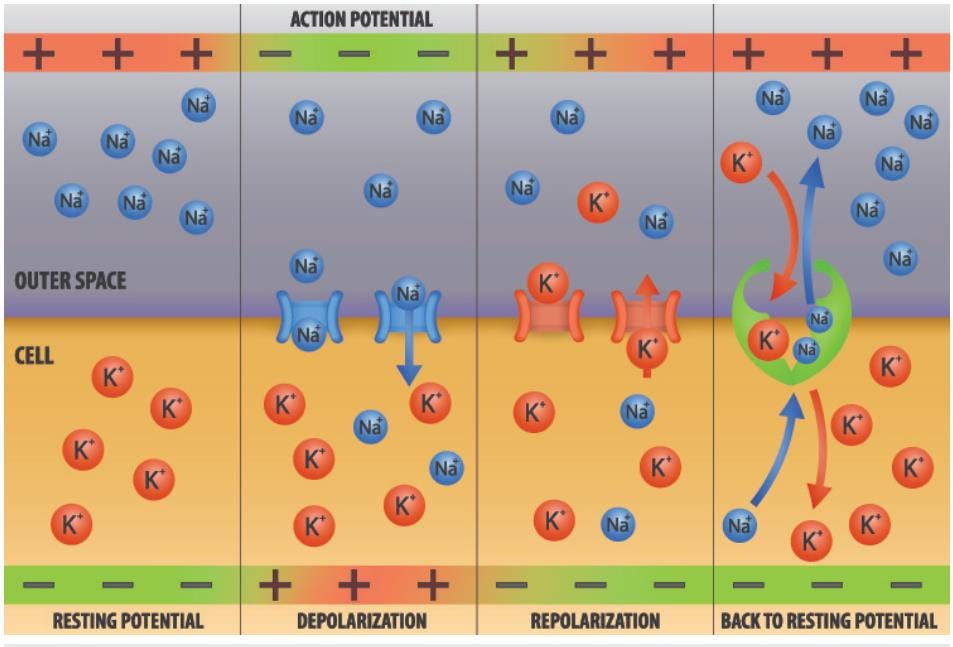
To clarify further, the process works this way, in several stages:
Depolarization: Sodium ions (Na⁺), which are more concentrated outside the neuron, flow into the neuron through these open channels. This influx of positive ions causes the inside of the neuron to become more positive, leading to depolarization. If the depolarization reaches a threshold (usually around -55 mV), it triggers an action potential.
Propagation: The depolarization of one segment of the neuron's membrane causes adjacent voltage-gated sodium channels to open. This creates a wave of depolarization that moves along the length of the neuron. As the action potential propagates, it moves like a domino effect along the nerve fibers (axons).
Repolarization: Shortly after the sodium channels open, they close and voltage-gated potassium channels open. Potassium ions (K⁺), which are more concentrated inside the neuron, flow out of the neuron. This outflow of positive ions restores the negative charge inside the neuron, leading to repolarization.
Hyperpolarization: The potassium channels are slow to close, causing an overshoot known as hyperpolarization, where the membrane potential becomes slightly more negative than the resting potential.
Return to Resting Potential: The neuron returns to its resting potential as the sodium-potassium pump restores the original ion concentrations by pumping sodium out of the neuron and potassium back in.
This sequence of depolarization and repolarization travels along the neuron, allowing the electrical signal to be transmitted rapidly and efficiently. The process ensures that the signal moves in one direction and allows the neuron to communicate with other neurons or target tissues, such as muscles or glands.
Ashcroft continues:
Another difference between the electrical signals in our heads and in our homes is their speed of transmission. An electrical signal in a wire travels at almost the speed of light, which is 186 thousand miles per second. It’s easy to see this, for when you flick the switch a light comes on at once, and telephones and the Internet provide almost instantaneous communication around the globe. By comparison, the fastest nerve impulses are pitifully slow, crawling along at a mere 0.07 miles a second (120 metres a second). Even the brightest of us cannot think at the speed of light. As well as being slower, the electrical impulses we generate are also much smaller. Your electric kettle needs three amps of current to run, but the currents that tell your heart when to beat are only a few millionths of an amp. Finally, while energy is needed in both cases, the power – the battery if you will – that drives the current is produced in quite different ways, as explained later. These differences between animal electricity and that which supplies our homes are simple to state, but took many years to understand. Although the fundamental properties of electricity were understood by the beginning of the nineteenth century, it is only in the last sixty years or so that we have begun to understand the origin of bioelectricity and only in the last fifteen years that we have had a glimpse of what the molecules (the ion channels) responsible for the electrical activity of our nerve and muscle cells actually look like. We are no more than a collection of cells, millions and millions of them – as many as the stars in the galaxy. They come in many different varieties, like muscle cells and brain cells and blood cells, and in multiple shapes and sizes, but they are all the same fundamental entity … [F]or understanding the electrical properties of cells we only need to consider the events at the cell surface, as it is here that voltage differences arise and nerve impulses are transmitted. Each of our cells is surrounded by a membrane that encloses its contents and serves as a barrier to the world outside, rather like the skin of a soap bubble. This membrane is made up of fats (technically known as lipids), which means that it is impermeable to most water-soluble substances. It arises from the simple fact that fats and water do not mix. As anyone who has made a vinaigrette salad dressing knows, over time the ingredients separate into a lower layer of vinegar with the lighter oil floating on top. The phospholipid molecules that make up the cell membrane have water-loving phosphate heads and lipid tails that prefer to avoid water, and they organize themselves into a double-layered membrane in which the water-shy lipid tails are sandwiched inside the bilayer between two layers of phosphate headgroups. Don’t think, though, that membrane lipids are as hard as butter – they are more the consistency of machine oil, so that the proteins that sit in them tend to float about and must be anchored to the cell’s cytoskeleton to keep them in their correct places.
The solutions inside our cells, and those of all other organisms on Earth, are high in potassium ions and low in sodium ions. In contrast, blood and the extracellular fluids that bathe our cells are low in potassium but high in sodium ions. These ionic differences are exploited to generate the electrical impulses in our nerve and muscle cells for, like water trapped behind a hydroelectric dam, they are an effective way of storing potential energy. Open the floodgates and that energy is instantly released as the ions redistribute themselves to try and establish equal concentrations on either side of the membrane. It is these ion movements that give rise to our nerve and muscle impulses.
A nice video showing this process can be found here (“Propagation of Action Potential”). It shows how positively charged ions push each other (same repels same) to the next ion channel, which reacts to the imbalance by opening its own channel, and so on down the line. Positive ions move from areas of low concentration to areas of high concentration due to electrochemical forces, specifically the electrochemical gradient. This movement is driven by the ions’ tendency to move toward regions of opposite charge and higher concentration, which helps to equalize the concentration imbalances across the membrane. Ion channels open or close in response to various stimuli, including changes in voltage across the cell membrane, binding of specific molecules (ligands) to the channel protein, mechanical force, and temperature. These stimuli trigger changes in the ion channel protein, leading to either opening or closing of the channel pore, which allows ions to flow across the membrane.
Back to Ashcroft:
The transmembrane sodium and potassium gradients are maintained by a minute molecular motor, known as the sodium pump, that spans the cell membrane. This protein pumps out excess sodium ions that leak into the cell and exchanges them for potassium ions. If the pump fails, the ion concentration gradients gradually run down and when they have collapsed completely no electrical impulses can be generated, in the same way that a flat battery cannot start your car. Consequently, your sense organs, nerves, muscles – indeed all your cells – simply grind to a halt. This is what happens when we die. As we no longer have the energy to power the sodium pump and maintain the ion differences across our cell membranes, our cells soon cease to function. And while externally applied electric shocks can interfere with the electrical impulses in our nerve and muscle cells, they cannot restore the ion concentration gradients across our cell membranes once they have collapsed. This, then, is why we cannot reanimate a corpse with electricity, and why the spark of life is different from the electricity supplied to our homes. Maintaining the ion gradients is expensive, for electricity does not come cheap, even when we produce it ourselves. It is extraordinary to think that about a third of the oxygen we breathe and half of the food we eat is used to maintain the ion concentration gradients across our cell membranes. The brain alone uses about 10 per cent of the oxygen you breathe to drive the sodium pump and keep your nerve cell batteries charged … How our cells come to be filled with potassium ions is something of a puzzle. The simplest explanation is that the first cells evolved in a solution high in potassium. Left to themselves, lipids spontaneously organize into liposomes, tiny fluid-filled spheres enclosed by a single skin of lipids. Such lipid films may have been the origin of the first membranes and the liposomes they gave rise to may have formed the precursors to real cells. Over three and a half billion years ago, we may imagine, liposomes engulfed self-replicating molecules such as RNA or DNA and so gave rise to the very first cells. The fluid enclosed within these first primitive cells would of necessity be the same as that which surrounded them. Thus the high internal potassium concentration characteristic of all cells – from the simplest bacterium to the most complex organism – may reflect the composition of the ancestral soup. This leaves a mystery. Where were those ancient waters rich in potassium? Currently, one popular view is that life evolved in the black smokers of the ocean floor, the hydrothermal vents that belch out superheated water rich in minerals. From a physiologist’s point of view, however, this seems rather unlikely, for the Precambrian seas were high in sodium, like those of today. Thus, I side with Charles Darwin, who suggested life evolved in a ‘warm little pond’; aeons ago, shallow puddles in which organic molecules could be concentrated, and into which potassium ions leached from the surrounding rocks or clays, may have been the birthplace of the first cells. At some point in the far distant past, single cells discovered that living together gave them a selective advantage and the first multicellular organisms were born. Because the extracellular solution that bathes our cells is high in sodium, it is likely that such early multicellular organisms evolved in the sea, which is largely a solution of sodium chloride (common salt). It is a fascinating idea that the solutions inside our cells, and those that make up our extracellular fluids, provide a fingerprint to our past history and help chart where life first evolved. The presence of a cell membrane brought numerous advantages. Molecules no longer diffused away from each other at random, but could be retained in close proximity within the cell and, more importantly, interact with one another. Cells could become specialized for different functions – evolving into muscle, liver and nerve cells, to name but a few. Like the walls of a mediaeval city, the membrane also protected the cell from toxins in its immediate environment and restricted substances from entering and leaving, because the lipids of which it is composed are impermeable to most substances. As a consequence, tightly guarded gates that enabled vital nutrients and waste products to enter and leave the cell became a necessity … [A]n ion channel is no more than a tiny protein pore. It has a central hole through which the ions move, and one or more gates that can be opened and closed as required to regulate ion movements. When the gate is open, ions such as sodium and potassium swarm through the pore, into or out of the cell, at a rate of over a million ions a second. Conversely, when the gate is closed, ion flux is prevented … A potassium channel, for example, will only let potassium ions through and excludes sodium and calcium ions, whereas a sodium channel allows sodium to permeate, but not potassium or calcium. Under resting conditions all cells have a voltage difference across their membrane, the inside of the cell usually being between 60 and 90 millivolts more negative than the outside. This resting potential arises because of a tug of war between the concentration and electrical gradients across the cell membrane that the potassium ion experiences. At rest, many potassium channels are open in the cell membrane. As potassium ions are high inside the cell but low outside, they rush out of the cell down their concentration gradient and, because potassium ions carry a positive charge, their exodus leads to a loss of positive charge – or, to put it another way, the inside of the cell becomes gradually more negative. At some point, the exit of potassium ions is impeded by the increasing negative charge within the cell, which exerts an attractive force on the potassium ion that counteracts further movement. The membrane potential at which the chemical force driving the potassium ions out of the cell and the electrical force holding them back exactly balance one another is known as the equilibrium potential. The importance of the resting potential is that it acts like a tiny battery in which electric charge (in the form of ion gradients) is separated by the insulating properties of the lipid membrane. This stored energy is used to power the electrical impulses of our nerve and muscle fibres … Ions take the path of least resistance and move down their concentration gradient from an area of high concentration to one of low concentration. The number of sodium ions is much higher outside the cell than inside, so that sodium ions flood into the cell when the sodium channel gates open. Conversely, as there are many more potassium ions inside than out, potassium ions tend to leave the cell when the potassium channels open. Because ions are charged, their flow produces an electric current. It is such currents, carried by ions surging through ion channels, that underlie all our nerve and muscle impulses, and that regulate the beating of our hearts, the movement of our muscles and the electrical signals in our brains that give rise to our thoughts. This, in essence, is how the energy stored in the concentration gradients is used to power the electrical impulses of our nerve and muscle fibres.
Ashcroft also explores the roles ion channels play in plants:
Almost all life on the planet depends on the ability of plants to capture the energy of the sun and store it as sugar molecules. This process, known as photosynthesis, is the ultimate source of all the food we eat, all the molecules from which our bodies are built, and most of the oxygen in the atmosphere. Photosynthesis involves the conversion of carbon dioxide and water into sugar and oxygen in a reaction powered by sunlight, and it takes place in specialized organelles, known as chloroplasts, that lie within plant cells. To prevent excessive water loss, the leaves of most plants are covered with a thick waxy cuticle. However, this also restricts the diffusion of oxygen and carbon dioxide into and out of the leaf, so that gas exchange can only take place via dedicated pores on the underside of the leaf, known as stomata, that act like microscopic windows … [M]ost plants balance photosynthesis and water stress by opening and closing their stomata throughout the day, as the ambient light and humidity conditions dictate. Stomata are composed of two ‘guard’ cells that both form the aperture of the pore and regulate its opening and closing by adjusting the amount of water they contain. When the guard cells are swollen and turgid the pore between them is forced open, whereas when they lose water and become flaccid the pore collapses shut. The water movements that influence guard cell volume, and thereby stomatal opening, are controlled by a combination of pumps and channels. An increase in light intensity causes positively charged hydrogen ions to be pumped out of the cell, creating a negative potential across the cell membrane. In turn, this change in membrane potential opens potassium channels, allowing potassium ions to enter the guard cells. Water follows the potassium ions, so that the guard cells swell by as much as 40 per cent, forcing open the stomatal pore. As long as the potassium channels remain open, the pore remains ajar. However, when light levels fall or the plant experiences water stress the potassium channels close. Consequently, water leaves the cell, the guard cells shrink and the stomatal pore closes. In a sense then, by controlling the turgidity of the guard cells, plant potassium channels regulate photosynthesis. Arguably, they are some of the most important ion channels on Earth. I find it strangely pleasing that these potassium channels belong to the same superfamily as the ones I am most passionate about. They must stem from a common ancestor that evolved long ago, before the animal and plant kingdoms divided.
And interestingly, and related to the previous essay series on the human immune system, Ashcroft writes: “Killer T-cells kill viruses and bacteria in a number of different ways, but one of them is by releasing perforins – ion channels that punch holes in alien cell membranes.”
In the next essay in this series, we’ll explore how our protein battery ion channels work, and the fascinating example of the electric eel.





Paul, I studied much of this in medical school physiology, but your coverage is infinitely better, more interesting, and more user friendly. So pleased you are doing this series. Thanks again.