Following on the previous essay series on our bodies’ means of communicating with electric current, in the following series of essays we’ll examine the other of the two main modes of communication within our bodies, namely the hormones estrogen in women, and testosterone in men.
First, we’ll examine estrogen in women through Cat Bohannon’s book Eve: How the Female Body Drove 200 Million Years of Human Evolution.
Bohannon first writes of how the added complexity of hormones in females makes its study relatively more difficult:
Many researchers default to male subjects for practical reasons: it’s difficult to control for the effects of female fertility cycles, particularly in mammals. A complex soup of hormones floods their bodies at regular intervals, whereas males’ sex hormones seem more stable. A good scientific experiment aims to be simple, designed with as few confounding factors as possible. As a postdoc in a Nobel laureate’s lab once told me, using males “just makes it easier to do clean science.” The variables, in other words, are easier to control, thereby making the data more interpretable with less work, and the results more meaningful. This is especially true for the complex systems involved in behavioral research, but can even be a problem with basic things like metabolism. Taking the time to control for the female reproductive cycle is considered difficult and expensive; the ovary itself is thought of as a “confounding factor.” So, unless a scientist is specifically asking a question about females, the female sex is left out of the equation. The experiments run faster, the papers come out sooner, and the researcher is more likely to get grant funding and tenure.
But that shouldn’t limit our interest in the hormone that drives half of humanity. As Bohannon continues:
Being sexed permeates every major feature of our mammalian bodies and the lives we live inside them, for mouse and human alike … I realized we needed a kind of user’s manual for the female mammal. A no-nonsense, hard-hitting, seriously researched (but readable) account of what we are. How female bodies evolved, how they work, what it really means to biologically be a woman … Eve traces the evolution of women’s bodies, from tits to toes, and how that evolution shapes our lives today. By piecing this evolution together and connecting it to recent discoveries, I hope to provide the latest answers to women’s most basic questions about their bodies … Whether we are in pain or joyful, abled or disabled, in sickness or in health until death do we disassemble, our bodies and the brains they contain are quite simply what we are. We are this flesh, these bones, this brief concordance of matter. From the way we grow our nails to the way we think, everything we call human is fundamentally shaped by how our bodies evolved. And because, as a species, we are sexed, there are critical things we should be thinking about when we talk about what it means to be Homo sapiens. We have to put the female body in the picture. If we don’t, it’s not just feminism that’s compromised. Modern medicine, neurobiology, paleoanthropology, even evolutionary biology all take a hit when we ignore the fact that half of us have breasts … Evolution works by building cheap upgrades on existing systems. Once one body feature is in place, that newly changed body interacts with its environment, and those interactions influence the rise of other features. Those new features lead to more changes, which often loop back and change the first feature: milk leads to nipples, and the caretaking habits involved in being a nursing mother help enable the development of the placental uterus. The placental uterus then influences our metabolism and the needs of our offspring, so breast milk starts to change. Breast milk changes, and eventually birth canals turn into petri dishes for the bacteria that help newborns digest sugary milk. In essence, the kid is coated on the way out with handy bugs that coevolved with our breast milk … Unraveling how each of our features really came to be gives us a better picture of what women are: one half of a very young, complex, and fascinating species … There’s no one mother of us all. Each system in our body is effectively a different age, not only because the cellular turnover rate differs between cell type and location (your skin cells are far younger than most of your brain cells, for instance), but also because the things we think of as distinct to our species evolved at different times and in different places. We don’t have one mother; we have many. And to each Eve, her particular Eden: We have the breasts we do because mammals evolved to make milk. We have the wombs we do because we evolved to “hatch” our eggs inside our own bodies. We have the faces we do, and our human sensory perception along with it, because primates evolved to live in trees. Our bipedal legs, our tool use, our fatty brains and chatty mouths and menopausal grandmothers—all of these traits that make us “human” came about at different times in our evolutionary past. In truth, we have billions of Edens, but just a handful of places and times that made our bodies the way they are. These particular Edens are often where we speciated: when our bodies evolved in ways that made us too different from others to be able to breed with them anymore. And if you want to understand women’s bodies, it’s largely these Eves and their Edens you need to think about. And so each chapter in this book will follow one of our defining features all the way back to its origins—its Eve, or sometimes Eves, and their Edens, from the damp swamps of the late Triassic to the grassy knolls of the Pleistocene.
Bohannon then moves to the “Eve” of mother’s milk, who she calls “Morgie,” and describes the importance of water to human survival:
[A]s far as the latest scientific research can determine, we make milk because we used to lay eggs and, weirdly, because we have a long-standing love affair with millions of bacteria. Unlike the Eves who came before her, Morgie nursed her young. Once they are born, newborn animals face four essential dangers: desiccation, predation, starvation, and disease. They can die of thirst. Something can eat them. They can starve to death. And if they manage to dodge all of those, they can still die from bacteria or parasites overwhelming their immune systems. Every mother in the animal world has evolved strategies to try to protect her offspring, but Morgie managed to combat all four by dousing her kids in stuff made of her own body. When we talk about breast milk, we usually describe it as a baby’s first food. The last thing you want to do is underfeed a baby, because a newborn needs fuel to build new fat and blood and bone and tissue. As a result, we usually assume newborns cry for milk because they’re hungry, but that is and isn’t true. The most important thing infants need after they are born is water. All living creatures, mammal or not, are mostly made of water. While the adult human body is 65 percent water, newborns are 75 percent. Most animals are essentially lumpy donuts filled with ocean. If you wanted to describe life on Earth in the simplest terms, you could say we’re energetic bags of highly regulated water. We use that water to transport molecules between cells, between organs, to splice molecules and build new ones, to fold proteins, to cushion our various lumps, to move nutrients and waste in the right directions. Our very DNA maintains its shape surrounded by carefully ordered molecules of water. An adult human can go without food for up to a month, but without water we die in three to four days. Any biologist will tell you that the story of life is really the story of water. Our earthly cells evolved in shallow oceans, and they never got over it. So newborn Earth animals need water as soon as possible. Fish drink constantly from the second they hatch. On land, slaking a newborn’s thirst is trickier. Some newborn reptiles are small enough that they can drink water droplets and absorb mist through their skin. Some seek out puddles and streams. Others, like newborn sea turtles, head straight for large bodies of water. But mammals seek the ocean in their mother’s abdomen; human breast milk is almost 90 percent water. Over time, ancient land mammals like Morgie evolved to slake their hatchlings’ thirst with milk. There are a number of advantages to this adaptation. For example, the newborns don’t have to move: the water comes to them. Pups of burrowing animals can stay in the safety of a small burrow a lot longer than creatures that need to get to water. Also, milk isn’t just water but a balance of water and minerals and other useful stuff. There were other advantages in replacing water with mother’s milk. Water is an ideal medium for transmitting disease. Think of Morgie’s body as the Jurassic world’s best water filter. Tiny, fragile newborns are especially susceptible to pathogens, in part because of their small size and in part because their newly independent immune systems are still developing. Morgie’s milk might have contained whatever pathogens she happened to be carrying, but it wouldn’t have introduced anything new to her pups. Her immune system could fight the good fight, until her pups were old enough to fight for themselves.
Bohannon then moves on the describe the history of human milk production in women:
Scientists think milk evolved to solve both the desiccation and the immunological problem in one go. But how it started—how the very first droplets of milk actually formed—that’s where the story takes an unexpected turn … Morgie needed to keep her eggs moist, but she also needed to keep them from becoming festering breeding grounds for waterborne bacteria and fungus. Most scientists assume her egg mucus contained a host of antifungal and antibacterial material as the sea turtle and platypus mothers’ mucus still does. When today’s leather-egged offspring are ready to hatch, they use a specially evolved tool (usually a sharp “egg tooth” that later falls out) to puncture the shell. Then they also lick up some of the egg-coating goo. Their first meal, in fact, is from the wet side of the eggshell. In all likelihood, this was the first mother’s milk: an egg-moistening mucus that Morgie’s grandmother secreted out of specialized glands near her pelvis. When her pups hatched, some of them licked up a bit of this extra stuff, which gave these offspring a serious evolutionary boost. By the time Morgie came along, these glands had evolved to secrete a goo containing more water, sugars, and lipids. Eventually they became “mammary patches” with specialized bits of fur over them that helped channel the gunk into the pups’ eager mouths … Early mammalian milk was probably a lot like modern women’s colostrum: a thick, yellowish, sticky-sweet ichor, super dense in immunological material and protein. For the first few days after a woman gives birth, her milk is incredibly special—a hot shot of immune system for her newborn baby. New mothers can find colostrum alarming, since it looks a bit like pus, but within a few days it converts to the bluish-white stuff we’re used to calling breast milk … [C]olostrum is especially dense with immunoglobulins: antibodies tagged to respond to pathogens that the mother’s body knows to be dangerous. In fact, before we discovered penicillin, cow colostrum was commonly used as an antibiotic. Colostrum doesn’t just boost the kid’s immune system by injecting antibodies; it’s also a reliable laxative, which is also crucial to building a baby’s immune system. On top of the thick yellow stuff coming out of her nipples, a new human mother might also be startled by what’s coming out of her little darling’s behind. Meconium, a baby’s first poop—actually first few poops—is thick, tarry, and alarmingly green-black. It doesn’t smell like much, thankfully, because it’s mostly broken-down blood, protein, and fluid the fetus ingested inside the womb. But it’s important that the stuff comes out fairly soon, and the laxative properties of colostrum help hurry that along—so well, in fact, that the intestines of a newborn drinking colostrum are wiped relatively clean. Which is precisely what needs to happen. Friendly bacteria—present in the mother’s milk, in her vagina, and on her skin—rapidly colonize a newborn’s intestines.
Bohannon then outlines the evolutionary development of the female uterus:
[S]omewhere in deep time, after the dawn of milk but before the Chicxulub apocalypse [that is, the time an asteroid collision with Earth caused the mass extinction of the dinosaurs], mammalian bodies started veering off the main road. Instead of laying their eggs, some number of ancient creatures started incubating them inside their bodies. Some of them became the marsupials, while others became eutherians like us—the placentals. We didn’t just keep our eggs warm in there; the entire female body became a gestation engine. I’m not sure it’s possible to sufficiently explain how insane this is. The majority of multicellular animals lay a clutch of eggs. Some of us let them loose in the ocean in a free-floating stream. Some of us tuck them safely away in a sticky glob. Some stay with the eggs, guarding them until they hatch. Others skip town. In other words, what animals do with our eggs varies widely. But laying eggs is normal. What’s not normal is letting eggs incubate and hatch inside your body, where they can do all kinds of catastrophic damage. What’s not normal is building a placenta and anchoring a developing fetus to the wall of the uterus. What’s not normal, in other words, is giving birth to live young. But that’s precisely what most mammals do, along with a very small number of unrelated fish and lizards. Thanks to the world-clearing burn and freeze of Chicxulub, gestating our young inside our bodies might have been a significant part of how our Eves managed to succeed. And all along the way, we carried our young inside our bodies instead of laying eggs like sensible creatures. This is why women’s bodies are built the way they are today. It’s a huge part of why our lives are the way they are: most women have periods, get pregnant, and give birth.
Bohannon makes some side comments on the clitoris:
[O]nly 25 percent of women of any age reliably experience an orgasm during vaginal intercourse. When you control for whether those women also experience any clitoral stimulation during sex, the numbers drop further. Despite their obvious evolutionary function as birthing tunnels and receptacles for sperm, the vagina isn’t the center of most women’s sexual satisfaction, old or young—that remains, hands down, the clitoris. If you use a No. 4 camel-hair paintbrush to stimulate a female rat’s clitoris, she’ll happily return to the place she associates with it, over and over and over. She’ll emit a series of subsonic squeaks while she’s there—a quiet lover she’s not—and both her brain and her behavior will show evidence of reward seeking and pleasure, and if you do it near an almond-scented pad, she’ll solicit sex from an almond-scented male later. Female rats who experience clitoral stimulation also show lowered stress and better general health than rats who don’t have that sort of stimulation. In other words, clitoral stimulation is good for a lab rat’s health, much as it seems to be for human women.
And then Bohannon discusses the fascinating evolutionary history of menstruation:
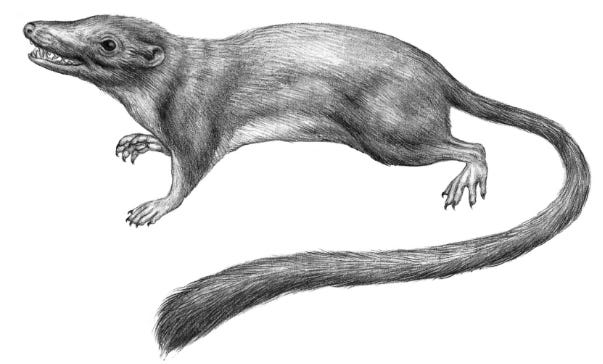
Shortly after the turn of the millennium, an international group of paleontologists and comparative biologists assembled a massive database of morphological features from all known living and extinct mammalian species. Then they used complex computation to trace everything they could think of backward through evolutionary time: from whence comes this particular jawbone, from whence those curious toes, from whence (importantly, for our purposes) these sorts of pelvic bones. About forty-five hundred characteristics, all told. They found that the last, true Eve of today’s eutherian mammals was almost certainly an arboreal insect eater, about the size of a modern squirrel, who spent most of her life climbing trees and snatching bugs from their high perches. She lived roughly sixty-six million years ago. Like many of our true Eves, we have no fossil that’s for sure the one. But we do have a creature with all the right traits dated within a useful margin of error that the researchers call Protungulatum donnae. Let’s call her Donna. Jubilant, the researchers even commissioned a rather adorable portrait of her for the paper: Her eyes, beady but blithe, shine black in the high forest light, where she stretches forward to snap up an insect. Her nose is large, her whiskers short, and her tail long and bushy tipped. There she is: our womb’s many-times-great-grand-rat.
Shedding menstrual material out of a vagina is super rare. And we’ve only just come up with a good theory for why we do it. Every month, the interior lining of the human uterus thickens. This is the endometrium, a layer of hillocked tissue thick with blood vessels, ready to nourish a freshly fertilized egg when it rolls down the fallopian tube and gently tumbles onto its soft bed. From there, the thickened endometrium—repurposed from a shell builder in deep evolutionary time—will create a network of blood vessels to nourish the growing placenta, and the pregnant woman will glow with satisfaction and eat chocolate-pickle ice cream and all will be right with the world. Or at least that’s what I learned in my eighth-grade health class … Refocusing on that simple fact—what the uterus does, rather than what men may or may not think about it—has led to a much more promising theory. The endometrium has two parts: the basal layer and the functional layer. The basal layer, clinging to the muscular interior of the uterine wall, isn’t shed every month. We shed only the functional layer, which is produced by the basal layer. When the right amount of estrogen rises in a woman’s bloodstream, the basal layer of the endometrium starts building up the functional layer that tops it, forming a spongy mass of mucous tissue and coiled blood vessels, riven by deep, narrow canals and tipped with a waving fringe of cilia. If a fertilized egg manages to hook onto the endometrium’s functional layer, it will start building a placenta. The functional layer of the uterus will then rapidly transform into what’s called the decidua, a thick buffer between the mother’s body and the growing embryo. Meanwhile, digging down into the decidua, the embryo will start building its part of the placenta. That’s right: the placenta is actually made of both embryonic tissue and the mother’s tissue—one of the only organs in the animal world made out of two separate organisms. One half is built from the blueprints in the embryo’s genetic matter. The other half, the placenta’s “basal plate,” grows out of the mother’s decidua. Two fleshy landscapes, one organ … If no fertilized egg is in the picture, the mother’s ovaries trigger a rise in progesterone after she ovulates, and the “functional layer” of the uterus breaks down and gets sloughed off. The uterus even helps out with minor contractions. If they’re severe enough, women experience these contractions as “cramps.”
As Bohannon explains, menstruation is actually an evolutionary defense mechanism carefully calibrated to help both the mother and the unborn child survive the experience of pregnancy:
The fact that menstrual material comes out of the vagina isn’t the most interesting part. The question is why the uterine lining starts building up before it knows a fertilized egg is barreling down the fallopian tubes toward it. Among Donna’s descendants, this trait is exceedingly rare. Yet it has evolved independently three different times: once for higher primates, once for certain bats, and once for the elephant shrew. Why would the trait arise in such radically unrelated species? Does it serve some purpose? Is there anything, in other words, for human women to be grateful for in our otherwise- unwelcome monthly uterine awareness program? Not really. It turns out the mammalian uterus isn’t a lush pillow— it’s a war zone. And ours may be one of the deadliest. Human women menstruate because it’s part of how we manage to survive our bloodsucking demon fetuses. The fetus has long evolved to hoover massive quantities of blood and other resources through the placenta. The mother’s body, meanwhile, has longer evolved to… survive. We mammals aren’t like salmon. We don’t tend to die right after we lay eggs. We actually need to live at least long enough to breast-feed our offspring. And for social mammals— especially creatures like us, who often have lifelong supportive relationships with our children— the benefit of a parent’s survival to our offspring greatly outlasts their gestation period. The uterus and its temporary passenger are, in fact, in conflict: the uterus evolving to protect the mother’s body from its semi-native invader, and the fetus and placenta evolving to try to work around the uterine safety measures. If a certain set of genetic mutations makes the offspring generally stronger, slightly better developed, and better nourished when it exits the mother’s body, those genes will be selected for. If it kills the mother, of course, it loses the war. Likewise, if the mother’s self-defense mechanisms are too strong, they kill the baby and she won’t pass on her genes. When the stakes are this high, each “healthy pregnancy” is a temporary détente: a bloody stalemate that lasts, in our case, roughly nine months. Like many other mammals with highly invasive placentas, our apelike Eves evolved a strategy for survival. Instead of waiting for a bomb to land, we dig our defenses early. We build up our linings on a regular basis, long before they are needed to protect the mother against the never-ending hunger of a human embryo.
In the next essay in this series, we’ll explore how estrogen shapes many other characteristics of female humans.