Continuing our essay series on Brian Villmoare’s “Big History” book The Evolution of Everything: The Patterns and Causes of Big History, this essay will explore the origins of life on Earth.
As Villmoare writes:
All life on Earth descends from a common ancestor. We know this because all life is related – we all use the same genetic code (DNA), and the other building blocks of life (the amino acids) are identical. In fact, it is possible to determine relatedness between any two living things by just looking at how different their DNA is. So, even though you look nothing alike, you are related to a housefly, a dandelion, a mushroom, and even the bacteria that causes ear infections. All of this life comes from a single, ancient ancestor. Sometime, between 3.5 and 4 billion years ago, life originated, and as far as we know it only happened once. All life after that point is a result of slow changes over time through evolution. Because this was such a profound moment, it has long fascinated researchers. The earliest life that became preserved in fossils was a very simple single-celled organism that converted the Sun’s energy into growth using photosynthesis, much as plants and algae do today … But how did this life originate? To understand how life originated we have to think about what the world was like during the transition from the Hadean [Eon] to the Archaean [Eon]. At that time, long before the appearance of any plants, the world was hot and rocky, with large bodies of water. The Earth did not have a large envelope of gaseous oxygen (in the form of the O2 molecule), which is today a product of billions of years of photosynthesis. Rather, there was scant O2 but large amounts of methane, ammonia and water. Methane and ammonia are organic molecules, which means that they have carbon, but in these molecules is also nitrogen and hydrogen (these same chemicals are found in all living organisms today). The conditions around 3.6 billion years ago – a mixture of relatively simple molecules sitting atop a relatively hot planet Earth – are known as the primordial soup. At some point, these chemicals started forming self-replicating structures. But these earliest structures left no trace, at least as far as we know.
We don’t know exactly how life spontaneously arose from the chemical stew of the early Earth, but as Villmoare writes:
By the middle of the twentieth century our understanding of early Earth was sufficient to allow us to try to figure out how life could originate from something nonliving, and scientists attempting to understand how life originated have conducted a series of experiments attempting to replicate the earliest appearance of life. The most famous of these experiments is the Miller–Urey experiments of 1952. In this series of experiments, the researchers put ammonia, methane, water, and hydrogen in a flask, heated the flask, and then ran an electrical current through the gas. This roughly replicated the conditions on early Earth, in which a hot surface temperature was likely often subject to electrical storms, injecting electrical energy into the primordial soup. They checked the condensed gas the next day and found amino acids. Amino acids are the ultimate product of DNA, and the bodies of all living things are composed of amino acids … [I]t is important to emphasize that even though Miller and Urey found a way to generate some of the components of life in their experiments, they did not actually generate life. Subsequent research by Miller and Urey, as well as quite a few other scientists, has not yet been able to make an actual life-form from simple chemical precursors.
Although we don’t know exactly how life came about, the odds of a spontaneous life occurrence weren’t zero:
Earth also had an enormous amount of time and space to experiment – most of the Earth was probably under water for hundreds of millions of years, subject to energy provided by lightning, volcanoes, and underground heat from the mantle radiating up, in which there were just lots of opportunities for chemicals to mix under various circumstances.
In any event:
All life appears to be the result of a single event, some 3.8 billion years ago. Everything that lives today, or has ever lived, traces its origin back to that single moment when life started. That is why all living things – bacteria, plants, animals, fungi – share the same genetic code; our DNA is made up of the same components, and they produce the same amino acids and proteins using the same processes … But researchers have not yet been able to “produce life in a test tube.” Why not? One factor is likely to be that we do not yet understand the precise geological and atmospheric conditions when life appeared. Also, life has only appeared once on Earth, and whatever the chemical conditions generated early life, it was clearly an unusual event. If life were easy to produce, we would see multiple strains of life, unrelated to each other. That is not the case – there is only one. But one advantage that nature has is time and space. Miller and Urey conducted their experiment in a half-liter of water over a few weeks. Life on Earth had a much bigger laboratory - it evolved somewhere over the entire expanse of the planet, over hundreds of millions of years. Some relatively unlikely chemical event occurred, but much like going fishing every day for years, rather than just one morning, increases your chance of catching a fish, having trillions of gallons of water over hundreds of millions of years, subject to thousands of volcanoes and millions of lightning storms, increases the chance of a rare event occurring.
Still:
[S]ome scientists argue that life probably came from somewhere else. Under this model (known as Panspermia), life may have originated in distant galaxies billions of years ago. Over time, meteors crashing into life-bearing planets would knock off chunks of the planets. These chunks, now meteors in their own right, could carry simple life (such as bacteria) wrapped in ice across the Universe. These meteors could travel into new solar systems, randomly seeding the planets in the “habitable zone” with life. Experiments by NASA and other groups have demonstrated that bacteria can survive extended periods of time in space … In 1969 the Murchison Meteorite fell on southern Australia … [It contained] uracil. Uracil is part of the genetic code known as RNA, which is closely related to DNA. So, whether or not the compounds found on the Murchison Meteorite are the product of extraterrestrial life, it certainly appears that the chemical processes associated with the appearance of life are not uncommon throughout the Universe. Interestingly, that meteorite appears to have been eroded by liquid water at some time in its past.
Now, what exactly is “life”? As Villmoare writes:
When something dies it not only stops its growth and movement, but, over time, it tends to return to the simple elements of which it is composed. It you have ever seen a dead animal, you know that after a fairly short time it will decompose and no longer be organized into the structures of which it was composed when it was alive. So, in effect, it is going from a highly organized state (with cells, organs, etc.) to a less organized state. This process, by which objects go from a highly organized state to a less organized state, is known as entropy. But things that are alive go against this process, taking simple chemicals and organizing them into physical structures. And they consolidate energy, in the form of their organized materials (think of the energy-dense fats or sugars in all living things). In 1875 Ludwig Boltzmann offered what I regard as the best overall definition of life: “The general struggle for existence of animate beings is not a struggle for raw materials – these, for organisms, are air, water and soil, all abundantly available – nor for energy which exists in plenty in any body in the form of heat, but a struggle for [negative] entropy, which becomes available through the transition of energy from the hot sun to the cold earth.” For him, life was defined as the use of energy to defeat the universal forces of entropy. The reason this is such a good definition is that it truly captures the essence of life – it is a struggle against the Universe, which seeks to reduce energy and order. Life seeks to capture energy and use it to create order. And this may be what is so unique and special about life – it resists the raw destructive forces of entropy.
Water helps life resist entropy by facilitating order:
Why is water necessary for life? The first part of the answer is that life needs fluid. Chemicals do not combine very well, nor nearly as completely, in their solid form. Think for a minute about two chemical elements that need to combine to make life. How do they find each other? If they are just solid pieces of rock, they probably won’t. They may just sit on the surface of the planet for billions of years. But if they are mixed into a fluid, they will inevitably find each other. Imagine a big glass of water. If you put a drop of blue food coloring in on one side, and a drop of red on the other, those two colors do not stay separate for long. After a while, the colors will combine. This is because of the way molecules in a fluid state move – they have a great deal of energy, and just keep bouncing around off each other. Given enough time, every molecule will bounce off every other molecule. But why water and not some other fluid? What is so special about H20? … [T]he water molecule has polarity: the hydrogen atoms, which are positively charged, are on one side of the molecule, while the negatively charged oxygen atom is on the other. When something is placed in water, the two different electrical charges tug differentially on the different components of whatever substance is in the water, and can literally pull it apart. This ability to dissolve substances means water is excellent for nature to experiment with mixing various chemicals [and also for cleaning things -- by pulling things that are either positively or negatively charged off things that are washed] … [O]nce life has formed, especially complex life, having water in the bodies of living things provides a way for the stable transport of other chemicals to get around the body.
In previous essays, we explored how evolution worked with the laws of physics to produce the processes necessary for life. As Villmoare elaborates:
The chemical organization of the bits of data in the coils of DNA is such that each of the two strands of the double helix is a mirror of the other, so the data of one strand are duplicated in the other. This would seem to be inefficient, but the “doubleness” of the DNA actually protects it. By having a mirror, the bits of DNA are bonded to other DNA, with no molecules available for bonding with random chemicals floating by. It makes the DNA strand, and the data stored in it, stable. Perhaps even more importantly, the mirrored side provides the template for repair. This stability, and the fidelity of the genetic code, is important, since our DNA is used millions or billions of times over our lifetimes. Any error would be replicated many, many times over.
Once this DNA-based replication method developed, it led to the first successful living organism — algae:
The earliest life on Earth that was widely successful was cyanobacteria, also known as green algae. This is a very simple organism – a bacterium – that also engages in photosynthesis. For almost 2 billion years, this was the dominant life-form on Earth, and the long-term success of cyanobacteria changed the geology of the Earth itself, generating oxygen that is reflected in ancient layers of oxidized iron. This oxygen is also what made more complex life possible later and is the oxygen in our atmosphere today. From roughly 3.6 billion years ago until fewer than a billion years ago, this multicellular life had no competition. From its origin, it spread around the globe, reproducing and engaging in photosynthesis. For our purposes, this generation of oxygen was a critical element in the later evolution of multicellular life. For almost three billion years, cyanobacteria were generating oxygen around the world. Today we see evidence of this build-up in the iron bands in the Earth that have become rust (oxidized), and, up until 1.8 billion years ago, the oxygen formed bonds with elements and compounds in the earth and ocean and did not accumulate in the atmosphere. Oxygen readily forms molecular bonds, and this is one of the most important characteristics of oxygen. For example, it is one of the reasons we can absorb it so easily by breathing. We have iron in our blood (in the hemoglobin molecules) that bonds with oxygen coming in through our lungs, and can transport it to our cells very rapidly (iron is red in the presence of oxygen, which is why rust is reddish and our blood is red). Over time, the chemicals in the Earth became saturated with oxygen, and at that point the oxygen became free (that is, not having bonded with any other compound or element), existing in large quantities on the surface of the planet. This started with the oceans at around 2.4 billion years ago, and the atmosphere by roughly 0.85 billion years ago, and it is after this point that we have a concentrated build-up of atmospheric oxygen on Earth. The additional free oxygen in the oceans enabled the evolution of eukaryotes (organisms with nuclei) roughly 2.4 million years ago, but it was not until after 850 million years ago, with a build-up of atmospheric free oxygen, that multicellular organisms (metazoans), with their complex and oxygen-dependent structures, could evolve.
Incidentally, how do scientists date these ancient things that happened so long ago? As Villmoare explains:
[M]ost methods for dating rocks and bones use the principle of radioactive decay. Some atoms are inherently radioactive, which means that they are always ejecting subatomic particles. When one of these elements kick off a particle, it becomes a different element. If there is a rock composed of one of these unstable elements, over time more and more of the rock will change from the original element to the new one. Scientists know how fast this radioactive decay occurs (the half life of the unstable element), so when they want to date a rock, they look at how much of the original (parent) atoms are present relative to the number of new (daughter) atoms. The age of the rock can be determined by measuring the ratio of parent atoms to daughter atoms. When dating the oldest rocks on Earth, geologists look for zircons, which are small crystals that have tiny amounts of uranium when they originally form. Over time, this uranium decays into lead. So, to date the crystal they look at the ratio of uranium to lead (uranium-lead dating). Other atomic elements are also used for dating fossils and bones. In order to date the earliest human bones in East Africa, rocks in the associated layers of sediment are dated with argon-argon dating, in which an unstable isotope of argon decays into another, more stable isotope of argon. A similar principle applies to dating organic material such as bones with carbon dating. Some of the carbon in your bones is in a particular form, carbon-14 (14C), in which there are two more neutrons than in most radioactively stable and common form of carbon carbon-12 (12C). Every time you eat, you replenish the 14C, which comes into our bodies through plants, or animals that have eaten plants. But when an animal dies, it stops absorbing any 14C, and over time that 14C begins to decay into the more stable 12C. Scientists wanting to know how long ago the animal died can look at the ratio of 14C to 12C and know how old the bones are. The problem with carbon dating is that 14C has a very short half life (about 5,700 years), and after about 60,000 years there is so little 14C left that it cannot be measured. So if scientists find a bone or plant fragment with no measureable 14C, all they can say is that it is older than 60,000 years. Fortunately, the rock-dating methods, such as uranium-lead or potassium-argon, have half lives in the billions of years; so as long as there are radioactively unstable rocks in the sediment layers with the fossils, they can be dated.
In the next essay in this series, we’ll explore how life evolved from algae to everything else.

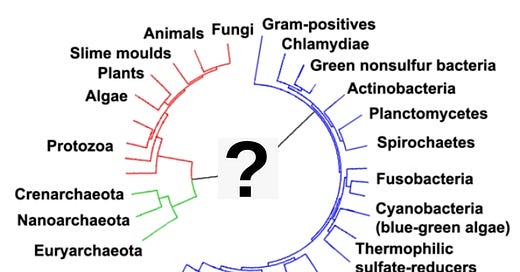

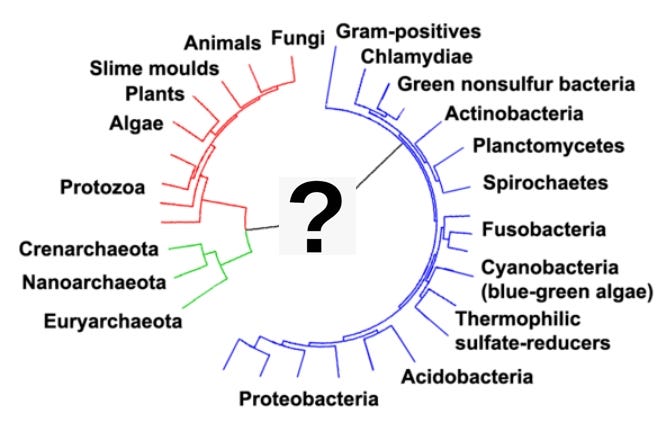
Life formed in a primordial soup is quite improbable.